In vivo neurophysiological assessment of in silico predictions of neurotoxicity: Citronellal, 3,4-dichloro-1-butene, and benzyl bromoacetate
- PMID: 35227730
- PMCID: PMC9133174
- DOI: 10.1016/j.neuro.2022.02.008
In vivo neurophysiological assessment of in silico predictions of neurotoxicity: Citronellal, 3,4-dichloro-1-butene, and benzyl bromoacetate
Abstract
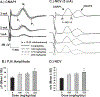
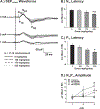
Neurotoxicants may be widespread in the environment and can produce serious health impacts in the human population. Screening programs that use in vitro methods have generated data for thousands of chemicals. However, these methods often do not evaluate repeated or prolonged exposures, which are required for many neurotoxic outcomes. Additionally, the data produced by such screening methods may not include mechanisms which play critical biological roles necessary for in vivo neurotoxicity. The Hard and Soft Acids and Bases (HSAB) in silico model focuses on chemical structure and electrophilic properties which are important to the formation of protein adducts. A group of structurally diverse chemicals have been evaluated with an in silico screening approach incorporating HSAB parameters. However, the predictions from the expanded chemical space have not been evaluated using in vivo methods. Three chemicals predicted to be cumulative toxicants were selected for in vivo neurotoxicological testing. Adult male Long-Evans rats were treated orally with citronellal (CIT), 3,4-dichloro-1-butene (DCB), or benzyl bromoacetate (BBA) for 8 weeks. Behavioral observations were recorded weekly to assess motor function. Peripheral neurophysiological measurements were derived from nerve excitability (NE) tests which involved compound muscle action potentials (CMAPs) in the tail and foot, and mixed nerve action potentials (MNAPs) in the tail. Compound nerve action potentials (CNAPs) and nerve conduction velocity (NCV) in the tail were also quantified. Peripheral inputs into the central nervous system were examined using somatosensory evoked potentials recorded from the cortex (SEPCTX) and cerebellum (SEPCEREB). CIT or BBA did not result in significant alterations to peripheral nerve or somatosensory function. DCB reduced grip-strength and altered peripheral nerve function. The MNAPs required less current to reach 50% amplitude and had a lower calculated rheobase, suggesting increased excitability. Increased CNAP amplitudes and greater NCV were also observed. Novel changes were found in the SEPCTX with an abnormal peak forming in the early portion of the waveforms of treated rats, and decreased latencies and increased amplitudes were observed in SEPCEREB recordings. These data contribute to testing an expanded chemical space from an in silico HSAB model for predicting cumulative neurotoxicity and may assist with prioritizing chemicals to protect human health.
Keywords: 3,4-Dichloro-1-butene; Benzyl bromoacetate; Citronellal; Hard and soft acids and bases; Nerve excitability; Neurophysiology.
Published by Elsevier B.V.
Conflict of interest statement
Figures




Similar articles
-
Carbon disulfide neurotoxicity in rats: VI. Electrophysiological examination of caudal tail nerve compound action potentials and nerve conduction velocity.Neurotoxicology. 1998 Feb;19(1):129-46. Neurotoxicology. 1998. PMID: 9498229
-
Application of the hard and soft, acids and bases (HSAB) theory as a method to predict cumulative neurotoxicity.Neurotoxicology. 2020 Jul;79:95-103. doi: 10.1016/j.neuro.2020.04.009. Epub 2020 May 5. Neurotoxicology. 2020. PMID: 32380191 Free PMC article.
-
Neurophysiological assessment of auditory, peripheral nerve, somatosensory, and visual system function after developmental exposure to gasoline, E15, and E85 vapors.Neurotoxicol Teratol. 2016 Mar-Apr;54:78-88. doi: 10.1016/j.ntt.2015.12.006. Epub 2015 Dec 22. Neurotoxicol Teratol. 2016. PMID: 26721698
-
Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry.Environ Health Perspect. 2012 Dec;120(12):1650-7. doi: 10.1289/ehp.1205432. Epub 2012 Oct 11. Environ Health Perspect. 2012. PMID: 23060388 Free PMC article. Review.
-
Neurophysiological impairments in multiple sclerosis-Central and peripheral motor pathways.Acta Neurol Scand. 2020 Nov;142(5):401-417. doi: 10.1111/ane.13289. Epub 2020 Jun 23. Acta Neurol Scand. 2020. PMID: 32474916
References
-
- Akaike H, 1973. Information theory as an extension of the maximum likelihood principle–In: Second International Symposium on Information Theory (Eds) Petrov BN, Csaki F. BNPBF Csaki Budapest: Academiai Kiado.
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.: Series B (Methodological) 57(1), 289–300.
-
- Benjamini Y, Hochberg Y, 2000. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educat. Behav. Stat 25(1), 60–83.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

