Immunogenicity and reactogenicity of homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimens in patients receiving maintenance dialysis
- PMID: 35227871
- PMCID: PMC8875769
- DOI: 10.1016/j.clim.2022.108961
Immunogenicity and reactogenicity of homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimens in patients receiving maintenance dialysis
Abstract
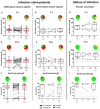
Patients receiving maintenance dialysis (MD) are vulnerable to COVID-19-related morbidity and mortality. Currently, data on SARS-CoV-2-specific cellular and humoral immunity post-vaccination in this population are scarce. We conducted a prospective single-center study exploring the specific cellular (interferon-γ and interleukin-2 ELISpot assays) and humoral immune responses (dot plot array and chemiluminescent microparticle immunoassay [CMIA]) at 4 weeks and 6 weeks following a single dose or a complete homologous dual dose SARS-CoV-2 vaccine regimen in 60 MD patients (six with a history of COVID-19). Our results show that MD patients exhibit a high seroconversion rate (91.7%) but the anti-spike IgG antibodies (CMIA) tend to wane rapidly after full immunization. Only 51.7% of the patients developed T cell immune response. High anti-spike IgG antibodies may predict a better cellular immunity. While patients with prior COVID-19 showed the best response after one, SARS-CoV-2-naïve patients may benefit from a third vaccine injection.
Keywords: Cellular immune response; Hemodialysis; Immunoglobulins; Peritoneal dialysis; T cells.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

References
-
- European Medicines Agency COVID-19 Vaccines. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr... Accessed July 25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

