Phosphoproteome Profiling of the Receptor Tyrosine Kinase MuSK Identifies Tyrosine Phosphorylation of Rab GTPases
- PMID: 35227894
- PMCID: PMC8972003
- DOI: 10.1016/j.mcpro.2022.100221
Phosphoproteome Profiling of the Receptor Tyrosine Kinase MuSK Identifies Tyrosine Phosphorylation of Rab GTPases
Abstract
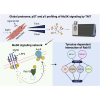
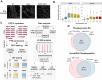
Muscle-specific receptor tyrosine kinase (MuSK) agonist antibodies were developed 2 decades ago to explore the benefits of receptor activation at the neuromuscular junction. Unlike agrin, the endogenous agonist of MuSK, agonist antibodies function independently of its coreceptor low-density lipoprotein receptor-related protein 4 to delay the onset of muscle denervation in mouse models of ALS. Here, we performed dose-response and time-course experiments on myotubes to systematically compare site-specific phosphorylation downstream of each agonist. Remarkably, both agonists elicited similar intracellular responses at known and newly identified MuSK signaling components. Among these was inducible tyrosine phosphorylation of multiple Rab GTPases that was blocked by MuSK inhibition. Importantly, mutation of this site in Rab10 disrupts association with its effector proteins, molecule interacting with CasL 1/3. Together, these data provide in-depth characterization of MuSK signaling, describe two novel MuSK inhibitors, and expose phosphorylation of Rab GTPases downstream of receptor tyrosine kinase activation in myotubes.
Keywords: Rab GTPases; c2c12; muscle-specific receptor tyrosine kinase; phosphoproteomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures





References
-
- Deenen J.C.W., Horlings C.G.C., Verschuuren J.J.G.M., Verbeek A.L.M., van Engelen B.G.M. The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J. Neuromuscul. Dis. 2015;2:73–85. - PubMed
-
- Herbst R. Musk function during health and disease. Neurosci. Lett. 2019;716:134676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

