Lorlatinib for advanced ROS1+ non-small-cell lung cancer: results of the IFCT-1803 LORLATU study
- PMID: 35227966
- PMCID: PMC9058895
- DOI: 10.1016/j.esmoop.2022.100418
Lorlatinib for advanced ROS1+ non-small-cell lung cancer: results of the IFCT-1803 LORLATU study
Abstract
Introduction: ROS1-rearranged (ROS1+) non-small-cell lung cancer (NSCLC) is a rare lung cancer with limited treatment options. Phase I-II studies with ROS1-tyrosine kinase inhibitors (TKIs) included small numbers of patients and real-world data are lacking. We investigate the efficacy and safety of lorlatinib, a third-generation TKI targeting ALK and ROS1, in patients with ROS1+ NSCLC treated through an expanded access program.
Methods: Consecutive patients with advanced ROS1+ NSCLC treated with lorlatinib between October 2015 and June 2019 were included. Data were collected from medical records. The primary endpoint was progression-free survival.
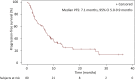
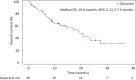
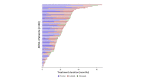
Results: Out of the 80 patients included, 47(59%) were female, 49(62%) never smokers (less than 100 cigarettes over the lifetime), and 68(85%) had stage IV NSCLC at diagnosis. Most frequent histology was adenocarcinoma (95%) and median age was 58.2 years. At the time of lorlatinib initiation, 51(64%) patients had brain metastases and 55(81%) were PS 0-1. Lorlatinib was administered as second/third/fourth/fifth+ line in 29%/28%/18%/26% of patients. All patients previously received at least one ROS1 TKI, and 55(69%) previously received chemotherapy. Median follow-up from lorlatinib initiation was 22.2 months. Median progression-free survival and overall survival from lorlatinib initiation were 7.1 months [95% confidence interval (CI) 5.0-9.9 months] and 19.6 months (95% CI 12.3-27.5 months). Median duration of treatment with lorlatinib was 7.4 months (95% CI 6.5-13.1 months). Overall response and disease control rates were 45% and 82%, respectively. The central nervous system response rate was 72%. Treatment was stopped due to toxicity in 10 patients (13%). The safety profile was consistent with previously published data.
Conclusions: Lorlatinib is a major treatment option for advanced refractory ROS1+ NSCLC in treatment strategy.
Keywords: NSCLC; ROS1; brain metastases; chemotherapy.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Role of the funder The funding source had no role in the design, data collection, analysis, or interpretation of the study, or in the preparation of this manuscript. Disclosure SB reports non-financial support from Lilly, GlaxoSmithKline, Roche, Pfizer, personal fees from Roche, Boehringer Ingelheim, grants from Intergroupe Francophone de Cancérologie Thoracique. BB reports grants from Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, Bristol Myers Squibb (BMS), Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Inivata, Janssen, Onxeo, OSE Immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, Tolero Pharmaceuticals. DMS reports grants from Pfizer, Roche, AstraZeneca, BMS, Merck Sharp & Dohme (MSD), personal fees from Pfizer, Roche, Takeda, AstraZeneca, Lilly, BMS, MSD, Novartis, Amgen, Abbvie, Becton Dickinson, and non-financial support from Pfizer, Roche, Takeda, AstraZeneca, BMS, MSD. JC reports personal fees from Pfizer, Roche, Takeda, Novartis, AstraZeneca, MSD, BMS, and Boehringer Ingelheim. VW reports honoraria from Roche, AstraZeneca, BMS, MSD and non-financial support from Roche, Pfizer. BR reports grants or contracts from Chugai, consulting fees from BMS, AstraZeneca, Roche, support for attending meetings and/or travel from BMS, Amgen, MSD, Roche. JB reports personal fees for advisory boards and educational symposia from AstraZeneca, Bayer, BMS, MSD, Roche, Daichii, and Servier. All other authors have declared no conflicts of interest.
Figures


References
-
- Lim S.M., Kim H.R., Lee J.-S., et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

