Combining predictive markers for severe COVID-19: Torquetenovirus DNA load and SARS-CoV-2 RNAemia
- PMID: 35227970
- PMCID: PMC8861125
- DOI: 10.1016/j.jcv.2022.105120
Combining predictive markers for severe COVID-19: Torquetenovirus DNA load and SARS-CoV-2 RNAemia
Abstract
Rationale/objectives: SARS-CoV-2 is the cause of worldwide COVID-19, which severity has been linked to the immune and inflammatory response. Here, we investigate Torquetenovirus (TTV) DNA load - a marker reflecting the intensity of the overall immune response - as well as SARS-CoV-2 RNAemia and IgM/IgG antibodies in COVID-19-positive patients.
Methods: Two hundred and fifteen COVID-19-positive patients were enrolled, including 87 severe cases and 128 mild-moderate cases. SARS-CoV-2 RNAemia and IgM/IgG antibodies, as well as TTV DNA loads, were measured on longitudinal plasma samples.
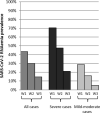
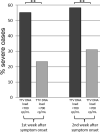
Results: The rate of severe cases was higher in patients with low TTV DNA load in plasma considering a threshold of 700 copies/mL. In severe patients, SARS-CoV-2 RNAemia positivity rates were higher than those in mild-moderate cases at any timepoint. When combined, TTV DNA load and SARS-CoV-2 RNAemia allowed to predict the outcome of COVID-19 infection, with a higher risk (HR=12.4) of ICU admission in patients with low TTV DNA load and positive SARS-CoV-2 RNAemia.
Conclusions: TTV DNA load and SARS-CoV-2 RNAemia may be effective, non-invasive markers reflecting disease severity and poor outcome that could be conveniently measured in a clinical laboratory setting, as soon as COVID-19 diagnosis is made.
Keywords: Biomarker; COVID-19; Immunity.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Gold J.A.W. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, march 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cité 7 juin 2020];69. Disponible sur: https://www.cdc.gov/mmwr/volumes/69/wr/mm6918e1.htm. - PMC - PubMed
-
- CDCMMWR. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, february 12–march 16, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cité 7 juin 2020]; 69. Disponible sur: https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm. - PMC - PubMed
-
- Jacobs J.L., Bain W., Naqvi A., Staines B., Castanha P.M.S., Yang H., et al. SARS-CoV-2 viremia is associated with COVID-19 severity and predicts clinical outcomes. Clin. Infect. Dis. Off Publ. Infect. Dis. Soc. Am. 2021;10:ciab686. août. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

