An organ-derived extracellular matrix triggers in situ kidney regeneration in a preclinical model
- PMID: 35228532
- PMCID: PMC8885654
- DOI: 10.1038/s41536-022-00213-y
An organ-derived extracellular matrix triggers in situ kidney regeneration in a preclinical model
Abstract
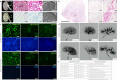
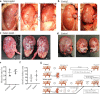
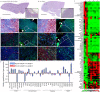
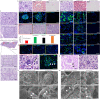
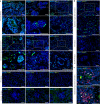
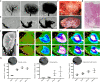
It has not been considered that nephrons regenerate in adult mammals. We present that an organ-derived extracellular matrix in situ induces nephron regeneration in a preclinical model. A porcine kidney-derived extracellular matrix was sutured onto the surface of partial nephrectomy (PN)-treated kidney. Twenty-eight days after implantation, glomeruli, vessels, and renal tubules, characteristic of nephrons, were histologically observed within the matrix. No fibrillogenesis was observed in the matrix nor the matrix-sutured kidney, although this occurred in a PN kidney without the matrix, indicating the structures were newly induced by the matrix. The expression of renal progenitor markers, including Sall1, Six2, and WT-1, within the matrix supported the induction of nephron regeneration by the matrix. Furthermore, active blood flow was observed inside the matrix using computed tomography. The matrix provides structural and functional foundations for the development of cell-free scaffolds with a remarkably low risk of immune rejection and cancerization.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr. Nephrol. 2011;26:1529–1533. - PubMed
-
- Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 2003;348:101–108. - PubMed
-
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr. Nephrol. 1987;1:385–392. - PubMed
-
- Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

