Inhibition of spleen tyrosine kinase decreases donor specific antibody levels in a rat model of sensitization
- PMID: 35228550
- PMCID: PMC8885754
- DOI: 10.1038/s41598-022-06413-2
Inhibition of spleen tyrosine kinase decreases donor specific antibody levels in a rat model of sensitization
Abstract
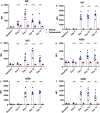
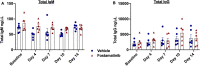
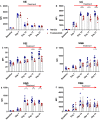
Antibody mediated rejection is a major cause of renal allograft loss. Circulating preformed donor specific antibodies (DSA) can result as a consequence of blood transfusion, pregnancy or prior transplantation. Current treatment strategies are limited due to partial or transient efficacy, adverse side-effects or patient unsuitability. Previous in vivo studies exploring autoimmune diseases have shown that spleen tyrosine kinase (SYK) signalling is involved in the development of pathogenic autoantibody. The role of SYK in allogenic antibody production is unknown, and we investigated this in a rodent model of sensitization, established by the transfusion of F344 whole blood into LEW rats. Two-week treatment of sensitized rats with selective SYK inhibitor fostamatinib strongly blocked circulating DSA production without affecting overall total immunoglobulin levels, and inhibition was sustained up to 5 weeks post-completion of the treatment regimen. Fostamatinib treatment did not affect mature B cell subset or plasma cell levels, which remained similar between non-treated controls, vehicle treated and fostamatinib treated animals. Our data indicate fostamatinib may provide an alternative therapeutic option for patients who are at risk of sensitization following blood transfusion while awaiting renal transplant.
© 2022. The Author(s).
Conflict of interest statement
FWKT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, MedImmune and Rigel Pharmaceuticals, and has consultancy agreements with Rigel Pharmaceuticals, Novartis and Baxter Biosciences, and is the Chief Investigator of an international clinical trial of a SYK inhibitor in IgA nephropathy (ClinicalTrials.gov NCT02112838) funded by Rigel Pharmaceuticals and a phase 2 clinical trial of fostamatinib in the treatment of chronic active antibody mediated rejection in renal transplantation, funded by Rigel Pharmaceuticals and Auchi Charitable Fund (EudraCT Number: 2018-000027-14). CDP has received a research project grant from GlaxoSmithKline and has a consultancy agreement with Genzyme. EM owns stock and is employed by Rigel Pharmaceuticals, Inc. CR reports personal fees from UCB, personal fees from Achillion Pharmaceutical, personal fees from Rigel Pharmaceuticals outside the submitted work. All other authors do not have competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

