The role of the anterior insular during targeted helping behavior in male rats
- PMID: 35228625
- PMCID: PMC8885669
- DOI: 10.1038/s41598-022-07365-3
The role of the anterior insular during targeted helping behavior in male rats
Abstract
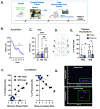
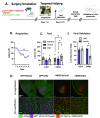
Empathy, the understanding of the emotional state of others, can be examined across species using the Perception Action Model, where shared affect promotes an action by "Observers" to aid a distressed "Target". The anterior insula (AI) has garnered interest in empathic behavior due to its role integrating sensory and emotional information of self and other. In the following studies, the AI was inhibited pharmacologically and chemogenetically during targeted helping. We demonstrate the insula is active during, and is necessary for the maintenance of, targeted helping. Analysis of ultrasonic vocalizations revealed distress calls from Targets increased when Observers' helping was attenuated due to insula inhibition. Targets' elevated distress was directly correlated to Observers' diminished helping behavior, suggesting emotional transfer between Observer and Target is blunted following Observer AI inhibition. Finally, the AI may selectively blunt targeted helping, as social exploration did not change in a social reward place conditioning task. These studies help further establish the anterior insula as a critical node in the empathic brain during targeted helping, even in the absence of direct social contact.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hoffman ML. Developmental synthesis of affect and cognition and its implications for altruistic motivation. Dev. Psychol. 1975;11(5):607–622. doi: 10.1037/0012-1649.11.5.607. - DOI
-
- de Waal FBM. The Age of Empathy: Nature’s Lessons for a Kinder Society. Three Rivers Press; 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

