Human topoisomerases and their roles in genome stability and organization
- PMID: 35228717
- PMCID: PMC8883456
- DOI: 10.1038/s41580-022-00452-3
Human topoisomerases and their roles in genome stability and organization
Abstract
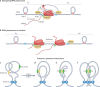
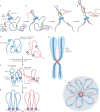
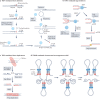
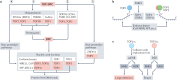
Human topoisomerases comprise a family of six enzymes: two type IB (TOP1 and mitochondrial TOP1 (TOP1MT), two type IIA (TOP2A and TOP2B) and two type IA (TOP3A and TOP3B) topoisomerases. In this Review, we discuss their biochemistry and their roles in transcription, DNA replication and chromatin remodelling, and highlight the recent progress made in understanding TOP3A and TOP3B. Because of recent advances in elucidating the high-order organization of the genome through chromatin loops and topologically associating domains (TADs), we integrate the functions of topoisomerases with genome organization. We also discuss the physiological and pathological formation of irreversible topoisomerase cleavage complexes (TOPccs) as they generate topoisomerase DNA-protein crosslinks (TOP-DPCs) coupled with DNA breaks. We discuss the expanding number of redundant pathways that repair TOP-DPCs, and the defects in those pathways, which are increasingly recognized as source of genomic damage leading to neurological diseases and cancer.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

