The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial
- PMID: 35228754
- PMCID: PMC8938265
- DOI: 10.1038/s41591-021-01659-1
The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial
Abstract
The sodium-glucose cotransporter 2 inhibitor empagliflozin reduces the risk of cardiovascular death or heart failure hospitalization in patients with chronic heart failure, but whether empagliflozin also improves clinical outcomes when initiated in patients who are hospitalized for acute heart failure is unknown. In this double-blind trial (EMPULSE; NCT04157751 ), 530 patients with a primary diagnosis of acute de novo or decompensated chronic heart failure regardless of left ventricular ejection fraction were randomly assigned to receive empagliflozin 10 mg once daily or placebo. Patients were randomized in-hospital when clinically stable (median time from hospital admission to randomization, 3 days) and were treated for up to 90 days. The primary outcome of the trial was clinical benefit, defined as a hierarchical composite of death from any cause, number of heart failure events and time to first heart failure event, or a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days, as assessed using a win ratio. More patients treated with empagliflozin had clinical benefit compared with placebo (stratified win ratio, 1.36; 95% confidence interval, 1.09-1.68; P = 0.0054), meeting the primary endpoint. Clinical benefit was observed for both acute de novo and decompensated chronic heart failure and was observed regardless of ejection fraction or the presence or absence of diabetes. Empagliflozin was well tolerated; serious adverse events were reported in 32.3% and 43.6% of the empagliflozin- and placebo-treated patients, respectively. These findings indicate that initiation of empagliflozin in patients hospitalized for acute heart failure is well tolerated and results in significant clinical benefit in the 90 days after starting treatment.
© 2022. The Author(s).
Conflict of interest statement
A.A.V. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. C.E.A. has received research/grant support and/or has been a consultant for Abbott, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, Vifor and German Federal Ministry of Education and Research. J.R.T. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics. S.P.C. is a consultant for Aiphia, Siemens, Bristol Myers Squibb, Boehringer Ingelheim and Vixiar and receives research support from the NIH, PCORI, AstraZeneca and Beckman Coulter. M.K. has received research grants from AstraZeneca and Boehringer Ingelheim, and has served as a consultant for AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi and Vifor. J.P.F. is a consultant for Boehringer Ingelheim and receives research support from AstraZeneca. M.E.N. has received speaking honoraria from Abbott, and is a consultant for Vifor, Roche and Amgen. J.T. is supported by the National University of Singapore Start-Up grant and has been a consultant and holds minor stocks for Us2.ai, and has received personal fees from Roche Diagnostics, Daiichi Sankyo, Boehringer Ingelheim and has a patent awarded for an ‘Automatic clinical workflow’ that recognizes and analyzes 2D and Doppler modality echocardiogram images for automated cardiac measurements. C.J.W.B. has received personal fees from AstraZeneca, Boehringer Ingelheim and Novartis. C.M. has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Johnson & Johnson and Pfizer. J.C.-C. has received unrestricted grants from Vifor and Novartis paid directly to his institute, and consulting fees from AstraZeneca, Bayer, and Boehringer Ingelheim. M.F. has received research grants from AstraZeneca, Novartis and Vifor Pharma; and fees as a speaker or consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Novo Nordisk, Novartis and Pharmacosmos. R.J.M. reports research support and personal fees from Boehringer Ingelheim, Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Y.S. reports consulting fees and honoraria from Boehringer Ingelheim. H.S. has received unrestricted grants from AstraZeneca paid directly to his institute, and consulting fees from Novartis and speaking fees from MSD, Pfizer, Sanofi and Amgen. M.S. has received personal payments from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Novartis. P.C.S. received honoraria and travel support from Bayer, AstraZeneca, Daiichi Sankyo, Novartis, Actelion, Roche, Sanofi Aventis, Pharmacosmos, Medtronic, Thoratec, Boehringer Ingelheim, Heartware, Coronus, Abbott, Edwards Inc., Boston Scientific, St. Jude Medical, Abiomed, and the German Cardiac Society. P.C.S. also received research support from the National Institute of Health (USA), the German Research Foundation, the Else Kröner Fresenius Foundation, German Heart Foundation, the European Society of Cardiology, Actelion, Medtronic, BMBF, Abiomed, Boehringer Ingelheim and Boston Scientific. P.C.S. served on advisory boards for the German Research Council, Eurotransplant, Novartis, Bayer, Pharmacosmos, AstraZeneca, Boehringer Ingelheim, the German Cardiac Society and the European Society of Cardiology. U.Z. received personal payments from AstraZeneca, Boehringer Ingelheim and Novartis. S.Z. has received consulting and or personal fees from Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Bayer, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Otsuka, Pfizer, Servier and Vifor. M.B. and A.S. are employees of Boehringer Ingelheim. J.P.B. is an employee of Elderbrook Solutions GmbH. P.P. reports personal fees from Boehringer Ingelheim, AstraZeneca, Servier, Bristol Myers Squibb, Amgen, Novartis, Merck, Pfizer, Berlin Chemie, and grants and personal fees from Vifor Pharma. J.B., M.A.P., S.P.J., R.G.K., L.S., M.V. and J.K.W. declare no competing interests.
Figures
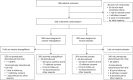

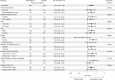
Comment in
-
Benefit of empagliflozin in acute heart failure.Nat Rev Cardiol. 2022 May;19(5):286. doi: 10.1038/s41569-022-00693-x. Nat Rev Cardiol. 2022. PMID: 35301454 No abstract available.
References
-
- Writing Group Members, Mozaffarian, D. et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation133, e38–360 (2016). - PubMed
-
- GBD Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

