Periosteum and development of the tissue-engineered periosteum for guided bone regeneration
- PMID: 35228996
- PMCID: PMC8858911
- DOI: 10.1016/j.jot.2022.01.002
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration
Abstract
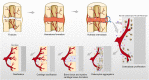
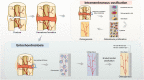
Background: Periosteum plays a significant role in bone formation and regeneration by storing progenitor cells, and also acts as a source of local growth factors and a scaffold for recruiting cells and other growth factors. Recently, tissue-engineered periosteum has been studied extensively and shown to be important for osteogenesis and chondrogenesis. Using biomimetic methods for artificial periosteum synthesis, membranous tissues with similar function and structure to native periosteum are produced that significantly improve the efficacy of bone grafting and scaffold engineering, and can serve as direct replacements for native periosteum. Many problems involving bone defects can be solved by preparation of idealized periosteum from materials with different properties using various techniques.
Methods: This review summarizes the significance of periosteum for osteogenesis and chondrogenesis from the aspects of periosteum tissue structure, osteogenesis performance, clinical application, and development of periosteum tissue engineering. The advantages and disadvantages of different tissue engineering methods are also summarized.
Results: The fast-developing field of periosteum tissue engineering is aimed toward synthesis of bionic periosteum that can ensure or accelerate the repair of bone defects. Artificial periosteum materials can be similar to natural periosteum in both structure and function, and have good therapeutic potential. Induction of periosteum tissue regeneration and bone regeneration by biomimetic periosteum is the ideal process for bone repair.
Conclusions: Periosteum is essential for bone formation and regeneration, and it is indispensable in bone repair. Achieving personalized structure and composition in the construction of tissue engineering periosteum is in accordance with the design concept of both universality and emphasis on individual differences and ensures the combination of commonness and individuality, which are expected to meet the clinical needs of bone repair more effectively.
The translational potential of this article: To better understand the role of periosteum in bone repair, clarify the present research situation of periosteum and tissue engineering periosteum, and determine the development and optimization direction of tissue engineering periosteum in the future. It is hoped that periosteum tissue engineering will play a greater role in meeting the clinical needs of bone repair in the future, and makes it possible to achieve optimization of bone tissue therapy.
Keywords: AF Antheraea pernyi fibroin, AMSCs adipose mesenchymal stem cells; BMP bone morphogenetic proteins, BMP-2 bone morphogenetic protein-2; BMSCs bone marrow stromal cell, CaPs calcium phosphate nanoparticles, COL I collagen I; Biomaterials; Bone defect healing; Bone repair; DOP dopamine, DSCs dental pulp stem cells; ECM extracellular matrix, GBR guided bone regeneration; GelMA methacrylate gelatin, HA hydroxyapatite; HAM human amniotic membrane, HCP human cultured periosteum; ICA Icariin, IGF-1 insulin-like growth factor-1; MBGNs mesoporous bioglass nanoparticles, MOX moxifloxacin hydrochloride; MSCs mesenchymal stem cells, n-HA nano-hydroxyapatite; OCN osteocalcin, OSX osterix; Osteogenesis; PCL polycaprolactone, PDCs periosteum derived cells; PDGF-BB platelet-derived growth factor-BB, PDO periosteal distraction osteogenesis; PEEK polyetheretherketone, PLA polylactic acid; PLLA l-lactic acid, PRP platelet-rich plasma; PU degradable polyurethane fibers without nano-hydroxyapatite, PUHA degradable polyurethane fibers with nano-hydroxyapatite; PVA polyvinyl alcohol, rhBMP-2 recombinant human bone morphogenetic protein-2; Periosteum; SEM scanning electron microscope, SF silk fibroin; SSP synthetic scaffold periosteum, TCP tricalcium phosphate; SiNPs Silica nanoparticles, SIS small intestinal submucosa; TGF-β transforming growth factor-β, VEGF vascular endothelial growth factors; Tissue-engineered periosteum; co-PUPCL a mixed fiber formed by PCL and polyurethane, DEX dexamethasone; rMSCs rat mesenchymal stem cells, Runx2 Runt-related transcription factor 2; s-PEEK sulfonated PEEK, SSCs skeletal stem cells.
© 2022 The Authors.
Conflict of interest statement
The authors have no conflict of interest relevant to this review.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

