Systemic and local immune responses to intraocular AAV vector administration in non-human primates
- PMID: 35229004
- PMCID: PMC8844404
- DOI: 10.1016/j.omtm.2022.01.011
Systemic and local immune responses to intraocular AAV vector administration in non-human primates
Abstract
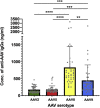
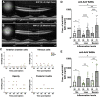
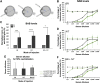
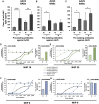
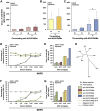
Positive clinical outcomes in adeno-associated virus (AAV)-mediated retinal gene therapy have often been attributed to the low immunogenicity of AAVs and immune privilege of the eye. However, several recent studies have shown potential for inflammatory responses. The current understanding of the factors contributing to inflammation, such as the pre-existence of serum antibodies against AAVs and their contribution to increases in antibody levels post-injection, is incomplete. The parameters that regulate the generation of new antibodies in response to the AAV capsid or transgene after intraocular injections are also insufficiently described. This study is a retrospective analysis of the pre-existing serum antibodies in correlation with changes in antibody levels after intraocular injections of AAV in non-human primates (NHPs) of the species Macaca fascicularis. In NHP serums, we analyzed the binding antibody (BAB) levels and a subset of these called neutralizing antibodies (NABs) that impede AAV transduction. We observed significantly higher pre-existing serum BABs against AAV8 compared with other serotypes and a dose-dependent increase in BABs and NABs in the serums collected post-injection, irrespective of the serotype or the mode of injection. Lastly, we were able to demonstrate a correlation between the serum BAB levels with clinical grading of inflammation and levels of transgene expression.
Keywords: AAVs; BABs; NABs; adeno-associated viruses; binding antibodies; gene therapy; neutralizing antibodies; non-human primates.
© 2022 The Authors.
Conflict of interest statement
D.D. is an inventor on a patent of AAV virions with variant capsid and methods of use thereof with royalties paid to Adverum Biotechnologies (WO2012145601 A2) and on patent applications on non-invasive methods to target cone photoreceptors (EP17306429.6 and EP17306430.4) licensed to Gamut Tx (now SparingVision). D.D. is a founder of Gamut Tx (now SparingVision). All other authors declare no competing interests.
Figures


References
-
- Wu Z., Asokan A., Samulski R.J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

