Characterization of AAV-mediated dorsal root ganglionopathy
- PMID: 35229008
- PMCID: PMC8851102
- DOI: 10.1016/j.omtm.2022.01.013
Characterization of AAV-mediated dorsal root ganglionopathy
Abstract
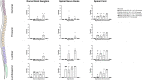
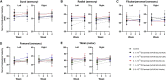
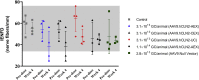
Recent studies in non-human primates administered recombinant adeno-associated viruses (rAAVs) have shown lesions in the dorsal root ganglia (DRG) of unknown pathogenesis. In this study, rAAV9s manufactured using different purification methods alongside a non-expressing Null AAV9 vector was administered to groups of cynomolgus monkeys followed by neuropathological evaluation after 4 weeks. Lesions, including neuronal degeneration, increased cellularity, and nerve fiber degeneration, were observed in the DRG, regardless of purification methods. Animals did not develop any neurological signs throughout the study, and there was no loss of function observed in neuro-electrophysiological endpoints or clear effects on intraepidermal nerve fiber density. However, magnetic resonance imaging (MRI) of animals with axonopathy showed an increase in short tau inversion recovery (STIR) intensity and decrease in fractional anisotropy. In animals administered the Null AAV9 vector, DRG lesions were not observed despite vector DNA being detected in the DRG at levels equivalent to or greater than rAAV9-treated animals. This study further supports that DRG toxicity is associated with transgene overexpression in DRGs, with particular sensitivity at the lumbar and lumbosacral level. The data from this study also showed that the nerve fiber degeneration did not correlate with any functional effect on nerve conduction but was detectable by MRI.
Keywords: AAV; AAV9; DRG; MRI; axonopathy; cynomolgus monkey; toxicity.
© 2022 The Author(s).
Conflict of interest statement
N.B., M.H., K.H.K., E.B., L.Y., Y.L., O.D., and M.F. are currently employees of REGENXBIO Inc. L.L. was an employee of StageBio at the time of study and is currently an employee of Charles River Laboratories; D.C. is currently an employee of Invicro, A Konica Minolta Company; M.M. is an employee of Preclinical Electrophysiology Consulting, LLC; J.Z. and E.A. were employees of Northern Biomedical Research at the time of study; and M.B. is an employee of StageBio.
Figures





References
-
- Kuzmin D.A., Shutova M.V., Johnston N.R., Smith O.P., Fedorin V.V., Kukushkin Y.S., van der Loo J.C.M., Johnstone E.C. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Discov. 2021;20:173–174. - PubMed
-
- Bolt M.W., Brady J.T., Whiteley L.O., Khan K.N. Development challenges associated with rAAV-based gene therapies. J. Toxicol. Sci. 2021;46:57–68. - PubMed
-
- Hordeaux J., Buza E.L., Dyer C., Goode T., Mitchell T.W., Richman L., Denton N., Hinderer C., Katz N., Schmid R., et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 2020;31:808–818. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

