Temporal and Quantitative Transcriptomic Differences Define Sexual Dimorphism in Murine Postnatal Bone Aging
- PMID: 35229061
- PMCID: PMC8861981
- DOI: 10.1002/jbm4.10579
Temporal and Quantitative Transcriptomic Differences Define Sexual Dimorphism in Murine Postnatal Bone Aging
Abstract
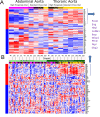
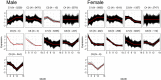
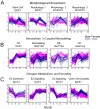
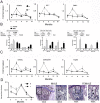
Time is a central element of the sexual dimorphic patterns of development, pathology, and aging of the skeleton. Because the transcriptome is a representation of the phenome, we hypothesized that both sex and sex-specific temporal, transcriptomic differences in bone tissues over an 18-month period would be informative to the underlying molecular processes that lead to postnatal sexual dimorphism. Regardless of age, sex-associated changes of the whole bone transcriptomes were primarily associated not only with bone but also vascular and connective tissue ontologies. A pattern-based approach used to screen the entire Gene Expression Omnibus (GEO) database against those that were sex-specific in bone identified two coordinately regulated gene sets: one related to high phosphate-induced aortic calcification and one induced by mechanical stimulation in bone. Temporal clustering of the transcriptome identified two skeletal tissue-associated, sex-specific patterns of gene expression. One set of genes, associated with skeletal patterning and morphology, showed peak expression earlier in females. The second set of genes, associated with coupled remodeling, had quantitatively higher expression in females and exhibited a broad peak between 3 to 12 months, concurrent with the animals' reproductive period. Results of phenome-level structural assessments of the tibia and vertebrae, and in vivo and in vitro analysis of cells having osteogenic potential, were consistent with the existence of functionally unique, skeletogenic cell populations that are separately responsible for appositional growth and intramedullary functions. These data suggest that skeletal sexual dimorphism arises through sex-specific, temporally different processes controlling morphometric growth and later coupled remodeling of the skeleton during the reproductive period of the animal. © 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: BONE AGING; SEX‐SPECIFIC; TEMPORAL TRANSCRIPTOMIC CLUSTER ANALYSIS.
© 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures


Similar articles
-
Age and Sex Differences in Load-Induced Tibial Cortical Bone Surface Strain Maps.JBMR Plus. 2021 Feb 16;5(3):e10467. doi: 10.1002/jbm4.10467. eCollection 2021 Mar. JBMR Plus. 2021. PMID: 33778328 Free PMC article.
-
The Cortical Bone Metabolome of C57BL/6J Mice Is Sexually Dimorphic.JBMR Plus. 2022 Jun 22;6(7):e10654. doi: 10.1002/jbm4.10654. eCollection 2022 Jul. JBMR Plus. 2022. PMID: 35866150 Free PMC article.
-
Different Responsiveness of Alveolar Bone and Long Bone to Epithelial-Mesenchymal Interaction-Related Factor.JBMR Plus. 2020 Jun 21;4(8):e10382. doi: 10.1002/jbm4.10382. eCollection 2020 Aug. JBMR Plus. 2020. PMID: 32803111 Free PMC article.
-
LGRs in Skeletal Tissues: An Emerging Role for Wnt-Associated Adult Stem Cell Markers in Bone.JBMR Plus. 2020 Jul 3;4(7):e10380. doi: 10.1002/jbm4.10380. eCollection 2020 Jul. JBMR Plus. 2020. PMID: 32666024 Free PMC article. Review.
-
Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors.Brain Res Brain Res Rev. 1993 Jan-Apr;18(1):51-74. doi: 10.1016/0165-0173(93)90007-m. Brain Res Brain Res Rev. 1993. PMID: 8467350 Review.
Cited by
-
Deletion of FNDC5/Irisin modifies murine osteocyte function in a sex-specific manner.bioRxiv [Preprint]. 2024 Mar 20:2023.11.06.565774. doi: 10.1101/2023.11.06.565774. bioRxiv. 2024. Update in: Elife. 2024 Apr 25;12:RP92263. doi: 10.7554/eLife.92263. PMID: 37986762 Free PMC article. Updated. Preprint.
-
Sexing Bones: Improving Transparency of Sex Reporting to Address Bias Within Preclinical Studies.J Bone Miner Res. 2023 Jan;38(1):5-13. doi: 10.1002/jbmr.4729. Epub 2022 Nov 13. J Bone Miner Res. 2023. PMID: 36301601 Free PMC article.
-
Deletion of FNDC5/irisin modifies murine osteocyte function in a sex-specific manner.Elife. 2024 Apr 25;12:RP92263. doi: 10.7554/eLife.92263. Elife. 2024. PMID: 38661340 Free PMC article.
-
Parathyroid hormone signaling in mature osteoblasts/osteocytes protects mice from age-related bone loss.Aging (Albany NY). 2021 Dec 30;13(24):25607-25642. doi: 10.18632/aging.203808. Epub 2021 Dec 30. Aging (Albany NY). 2021. PMID: 34968192 Free PMC article.
-
Collagen (I) homotrimer potentiates the osteogenesis imperfecta (oim) mutant allele and reduces survival in male mice.Dis Model Mech. 2022 Sep 1;15(9):dmm049428. doi: 10.1242/dmm.049428. Epub 2022 Sep 28. Dis Model Mech. 2022. PMID: 36106514 Free PMC article.
References
-
- Horbaly HE, Kenyhercz MW, Hubbe M, Steadman DW. The influence of body size on the expression of sexually dimorphic morphological traits. J Forensic Sci. 2019;64(1):52‐57. - PubMed
-
- Rowe DW, Adams DJ, Hong SH, et al. Screening gene knockout mice for variation in bone mass: analysis by μCT and histomorphometry. Curr Osteoporos Rep. 2018;16(2):77‐94. - PubMed
-
- Djuric M, Djonic D, Milovanovic P, et al. Region‐specific sex‐dependent pattern of age‐related changes of proximal femoral cancellous bone and its implications on differential bone fragility. Calcif Tissue Int. 2010;86(3):192‐201. - PubMed
-
- Gabet Y, Bab I. Microarchitectural changes in the aging skeleton. Curr Osteoporos Rep. 2011;9(4):177‐183. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
