Non-Obese MKR Mouse Model of Type 2 Diabetes Reveals Skeletal Alterations in Mineralization and Material Properties
- PMID: 35229063
- PMCID: PMC8861985
- DOI: 10.1002/jbm4.10583
Non-Obese MKR Mouse Model of Type 2 Diabetes Reveals Skeletal Alterations in Mineralization and Material Properties
Abstract
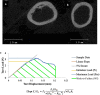
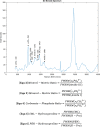
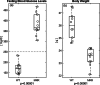
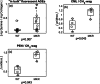
Obesity is a common comorbidity of type 2 diabetes (T2D). Therefore, increased risk of fragility fractures in T2D is often confounded by the effects of obesity. This study was conducted to elucidate the mechanistic basis by which T2D alone leads to skeletal fragility. We hypothesized that obesity independent T2D would deteriorate bone's material quality by accumulating defects in the mineral matrix and undesired modifications in its organic matrix associated with increased oxidative stress and hyperglycemia. To test this hypothesis, we used 15-week-old male non-obese mice with engineered muscle creatine kinase promoter/human dominant negative insulin growth factor 1 (IGF-I) receptor (MKR) and FVB/N wild-type (WT) controls (n = 12/group). MKR mice exhibit reduced insulin production and loss of glycemic control leading to diabetic hyperglycemia, verified by fasting blood glucose measurements (>250 mg/dL), without an increase in body weight. MKR mice showed a significant decrease in femoral radial geometry (cortical area, moment of inertia, cortical thickness, endosteal diameter, and periosteal diameter). Bone mineral density (BMD), as assessed by micro-computed tomography (μCT), remained unchanged; however, the quality of bone mineral was altered. In contrast to controls, MKR mice had significantly increased hydroxyapatite crystal thickness, measured by small-angle X-ray scattering, and elongated c-axis length of the crystals evaluated by confocal Raman spectroscopy. There was an increase in changes in the organic matrix of MKR mice, associated with enhanced glycoxidation (carboxymethyl-lysine [CML] and pentosidine) and overall glycation (fluorescent advanced glycation end products), both of which were associated with various measures of bone fragility. Moreover, increased CML formation positively correlated with elongated mineral crystal length, supporting the role of this negatively charged side chain to attract calcium ions, promote growth of hydroxyapatite, and build a physical link between mineral and collagen. Collectively, our results show, for the first time, changes in bone matrix in a non-obese T2D model in which skeletal fragility is attributable to alterations in the mineral quality and undesired organic matrix modifications. © 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: BONE QUALITY; GLYCATION; MINERALIZATION; NON‐OBESE; TYPE 2 DIABETES.
© 2021 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures





References
-
- Vaag A. On the pathophysiology of late onset non‐insulin dependent diabetes mellitus. Current controversies and new insights. Dan Med Bull. 1999;46(3):197‐234. - PubMed
LinkOut - more resources
Full Text Sources
