ATP Synthase K+- and H+-Fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: I. Characterization of Ion Fluxes
- PMID: 35229078
- PMCID: PMC8867323
- DOI: 10.1093/function/zqab065
ATP Synthase K+- and H+-Fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: I. Characterization of Ion Fluxes
Abstract
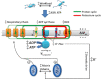
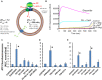
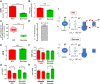
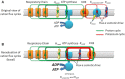
ATP synthase (F1Fo) synthesizes daily our body's weight in ATP, whose production-rate can be transiently increased several-fold to meet changes in energy utilization. Using purified mammalian F1Fo-reconstituted proteoliposomes and isolated mitochondria, we show F1Fo can utilize both ΔΨm-driven H+- and K+-transport to synthesize ATP under physiological pH = 7.2 and K+ = 140 mEq/L conditions. Purely K+-driven ATP synthesis from single F1Fo molecules measured by bioluminescence photon detection could be directly demonstrated along with simultaneous measurements of unitary K+ currents by voltage clamp, both blocked by specific Fo inhibitors. In the presence of K+, compared to osmotically-matched conditions in which this cation is absent, isolated mitochondria display 3.5-fold higher rates of ATP synthesis, at the expense of 2.6-fold higher rates of oxygen consumption, these fluxes being driven by a 2.7:1 K+: H+ stoichiometry. The excellent agreement between the functional data obtained from purified F1Fo single molecule experiments and ATP synthase studied in the intact mitochondrion under unaltered OxPhos coupling by K+ presence, is entirely consistent with K+ transport through the ATP synthase driving the observed increase in ATP synthesis. Thus, both K+ (harnessing ΔΨm) and H+ (harnessing its chemical potential energy, ΔμH) drive ATP generation during normal physiology.
Keywords: ATP synthesis; mitochondrial K+ transport; mitochondrial KATP channel; proteoliposomes; single molecule bioenergetics; unitary K+ currents.
Published by Oxford University Press on behalf of American Physiological Society 2021.
Figures



Comment in
-
Rethinking Mitchell's Chemiosmotic Theory: Potassium Dominates Over Proton Flux to Drive Mitochondrial F1Fo-ATP Synthase.Function (Oxf). 2022 Mar 9;3(2):zqac012. doi: 10.1093/function/zqac012. eCollection 2022. Function (Oxf). 2022. PMID: 35399493 Free PMC article. No abstract available.
-
Setting the Record Straight: A New Twist on the Chemiosmotic Mechanism of Oxidative Phosphorylation.Function (Oxf). 2022 Apr 19;3(3):zqac018. doi: 10.1093/function/zqac018. eCollection 2022. Function (Oxf). 2022. PMID: 35601666 Free PMC article. No abstract available.
References
-
- Cross RL, Muller V.. The evolution of A-, F-, and V-type ATP synthases and ATPases: reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 2004;576(1-2):1–4.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Stock D, Leslie AG, Walker JE.. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286(5445):1700–1705.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Kuhlbrandt W, Davies KM.. Rotary ATPases: a New Twist to an Ancient Machine. Trends Biochem Sci. 2016;41(1):106–116. - PubMed
-
- Boyer PD. The ATP synthase–a splendid molecular machine. Annu Rev Biochem. 1997;66(1):717–749.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE.. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370(6491):621–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

