Angiotensin II type 1 receptor localizes at the blood-bile barrier in humans and pigs
- PMID: 35229169
- PMCID: PMC9114028
- DOI: 10.1007/s00418-022-02087-z
Angiotensin II type 1 receptor localizes at the blood-bile barrier in humans and pigs
Abstract
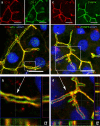
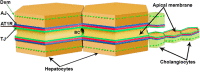
Animal models and clinical studies suggest an influence of angiotensin II (AngII) on the pathogenesis of liver diseases via the renin-angiotensin system. AngII application increases portal blood pressure, reduces bile flow, and increases permeability of liver tight junctions. Establishing the subcellular localization of angiotensin II receptor type 1 (AT1R), the main AngII receptor, helps to understand the effects of AngII on the liver. We localized AT1R in situ in human and porcine liver and porcine gallbladder by immunohistochemistry. In order to do so, we characterized commercial anti-AT1R antibodies regarding their capability to recognize heterologous human AT1R in immunocytochemistry and on western blots, and to detect AT1R using overlap studies and AT1R-specific blocking peptides. In hepatocytes and canals of Hering, AT1R displayed a tram-track-like distribution, while in cholangiocytes AT1R appeared in a honeycomb-like pattern; i.e., in liver epithelia, AT1R showed an equivalent distribution to that in the apical junctional network, which seals bile canaliculi and bile ducts along the blood-bile barrier. In intrahepatic blood vessels, AT1R was most prominent in the tunica media. We confirmed AT1R localization in situ to the plasma membrane domain, particularly between tight and adherens junctions in both human and porcine hepatocytes, cholangiocytes, and gallbladder epithelial cells using different anti-AT1R antibodies. Localization of AT1R at the junctional complex could explain previously reported AngII effects and predestines AT1R as a transmitter of tight junction permeability.
Keywords: AT1R; Canals of Hering; Gallbladder; Human liver; Porcine liver; Tight junctions.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures




Similar articles
-
Angiopoietin-like protein 2 expression is suppressed by angiotensin II via the angiotensin II type 1 receptor in rat cardiomyocytes.Mol Med Rep. 2016 Sep;14(3):2607-13. doi: 10.3892/mmr.2016.5544. Epub 2016 Jul 25. Mol Med Rep. 2016. PMID: 27483989 Free PMC article.
-
Essential role of angiotensin receptors in the modulation of intestinal epithelial cell apoptosis.J Pediatr Gastroenterol Nutr. 2013 Nov;57(5):562-9. doi: 10.1097/MPG.0b013e31829f1336. J Pediatr Gastroenterol Nutr. 2013. PMID: 23783021
-
Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system.J Mol Cell Cardiol. 2014 Oct;75:25-39. doi: 10.1016/j.yjmcc.2014.06.008. Epub 2014 Jun 27. J Mol Cell Cardiol. 2014. PMID: 24976017 Free PMC article.
-
Adrenal angiotensin II type 1 receptor biased signaling: The case for "biased" inverse agonism for effective aldosterone suppression.Cell Signal. 2021 Jun;82:109967. doi: 10.1016/j.cellsig.2021.109967. Epub 2021 Feb 25. Cell Signal. 2021. PMID: 33640432 Review.
-
Biased agonism/antagonism at the AngII-AT1 receptor: Implications for adrenal aldosterone production and cardiovascular therapy.Pharmacol Res. 2017 Nov;125(Pt A):14-20. doi: 10.1016/j.phrs.2017.05.009. Epub 2017 May 13. Pharmacol Res. 2017. PMID: 28511989 Review.
Cited by
-
Endothelial Dysfunction and Liver Cirrhosis: Unraveling of a Complex Relationship.Int J Mol Sci. 2024 Nov 29;25(23):12859. doi: 10.3390/ijms252312859. Int J Mol Sci. 2024. PMID: 39684569 Free PMC article. Review.
-
In focus in HCB.Histochem Cell Biol. 2022 May;157(5):493-495. doi: 10.1007/s00418-022-02108-x. Histochem Cell Biol. 2022. PMID: 35513613 No abstract available.
-
Comparative microanatomical and histochemical biodistribution profiles of different types of mucins in the intestinal mucosa of some tetrapod representatives.J Mol Histol. 2022 Apr;53(2):449-472. doi: 10.1007/s10735-022-10071-z. Epub 2022 Mar 6. J Mol Histol. 2022. PMID: 35249181
References
-
- Afroze SH, Munshi MK, Martinez AK, Uddin M, Gergely M, Szynkarski C, Guerrier M, Nizamutdinov D, Dostal D, Glaser S. Activation of the renin–angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 2015;308:G691–701. doi: 10.1152/ajpgi.00116.2014. - DOI - PMC - PubMed
-
- Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolas JM, Jimenez W, Weich N, Gutierrez-Ramos JC, Arroyo V, Rodes J. Activated human hepatic stellate cells express the renin–angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117–125. doi: 10.1016/S0016-5085(03)00695-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- INST 1856/66-1 FUGG/Deutsche Forschungsgemeinschaft
- A/12/93239/German Academic Exchange Service-German Egyptian Research Long-Term scholarship
- 91541390/German Academic Exchange Service-German Egyptian Research Long-Term scholarship
- N14/Graduate Program in Pharmacology and Experimental Therapeutics of the University of Cologne and Bayer Healthcare
LinkOut - more resources
Full Text Sources
Other Literature Sources

