ATP citrate lyase controls hematopoietic stem cell fate and supports bone marrow regeneration
- PMID: 35229328
- PMCID: PMC9016348
- DOI: 10.15252/embj.2021109463
ATP citrate lyase controls hematopoietic stem cell fate and supports bone marrow regeneration
Abstract
In order to support bone marrow regeneration after myeloablation, hematopoietic stem cells (HSCs) actively divide to provide both stem and progenitor cells. However, the mechanisms regulating HSC function and cell fate choice during hematopoietic recovery remain unclear. We herein provide novel insights into HSC regulation during regeneration by focusing on mitochondrial metabolism and ATP citrate lyase (ACLY). After 5-fluorouracil-induced myeloablation, HSCs highly expressing endothelial protein C receptor (EPCRhigh ) were enriched within the stem cell fraction at the expense of more proliferative EPCRLow HSCs. These EPCRHigh HSCs were initially more primitive than EPCRLow HSCs and enabled stem cell expansion by enhancing histone acetylation, due to increased activity of ACLY in the early phase of hematopoietic regeneration. In the late phase of recovery, HSCs enhanced differentiation potential by increasing the accessibility of cis-regulatory elements in progenitor cell-related genes, such as CD48. In conditions of reduced mitochondrial metabolism and ACLY activity, these HSCs maintained stem cell phenotypes, while ACLY-dependent histone acetylation promoted differentiation into CD48+ progenitor cells. Collectively, these results indicate that the dynamic control of ACLY-dependent metabolism and epigenetic alterations is essential for HSC regulation during hematopoietic regeneration.
Keywords: Acly; bone marrow regeneration; hematopoietic stem cells; mitochondrial metabolism.
© 2022 The Authors.
Figures
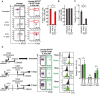
- A–C
The frequency of Sca‐1+c‐Kit+ cells (A) or Sca‐1+ cells (B) and c‐Kit expression levels (C), in the L‐ESLAM fraction before and after the administration of 5‐FU. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- D
According to the indicated schedule, 10,000 L−ESLAM HSCs (Ly5.2) were transplanted without irradiation into untreated or 5‐FU‐treated mice, and c‐Kit levels in the donor or recipient L−ESLAM fraction were subsequently examined. Numbers within each histogram of donor cells represent the number of CFSE+ cells detected. The graph depicts the relative MFI of c‐kit in the indicated cells. Data are shown as means ± SD (n = 4, two independent experiments, N.S.; not significant by the t‐test).

Kinetics of cell numbers in Lineage−EPCR+CD150+CD48− (L‐ESLAM) HSCs, Lineage−EPCR+CD150+CD48+ (CD150+CD48+) progenitor cells, and Lineage−EPCR+CD150−CD48+ (CD150−CD48+) progenitor cells after the administration of 5‐FU (250 mg/kg, i.v.). The small graph is an enlargement of 0 to 8 days after the administration of 5‐FU. (* or **) in the small graph represents the statistical analysis of HSC between 3 and 4 or 7 days, respectively. Data are shown as means ± SD (n ≥ 4, two or more independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
The enrichment plots of genes related to cell cycle in the comparison of the steady state with the early (upper) or late phase (lower). NES and q values represent normalized enrichment scores and FDR, respectively.
The frequency of EdC+ cells within L−ESLAM HSCs 24 h after the administration of EdC (150 mg/kg, i.p.). Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01 by the t‐test).
A principal component analysis (PCA) using the transcriptome data of L−ESLAM HSCs derived from untreated (steady state) or 5‐FU‐teated mice at 6 (early phase) or 10 days (late phase).
Comparison of gene expression patterns in HSCs between the early (6 days) and late phases (10 days) on GSEA. The graph shows normalized enrichment scores (NES). Bold and italic mean gene sets show (P < 0.05, FDR < 0.05) or (P < 0.05), respectively.
The frequencies of phenotypic HSCs (Orange gate) and CD48+ progenitor cells (blue gate) after a 4‐day culture of HSCs derived from 5‐FU‐treated mice (at 6–9 days). Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).

- A
An experimental model to examine divided HSCs within 5‐FU‐treated mice using the transplantation of dye (CFSE or CytoTellTMRed 650)‐labeled HSCs.
- B
The frequency of L−ESLAM HSCs within the divided fraction in 5‐FU recipients or undivided fraction in untreated recipients. Numbers within each histogram represent the CFSE+ cell number detected. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- C
Cell division number in donor‐derived cells within untreated or 5‐FU recipients. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, **P < 0.05, N.S.; not significant by the t‐test).
- D
The enrichment plots of cell cycle‐related gene sets in the comparison of the early phase with the late phase.
- E, F
Fifty L−ESLAM HSCs within the divided fraction of donor‐derived cells in 5‐FU recipients or undivided fraction in untreated recipients were transplanted along with 2 × 105 competitor cells. After 20 weeks, the chimerism of peripheral blood was examined. White or gray circles within plots represent donor cell chimerism in multilineage‐reconstructed recipient mice (> 0.5% for myeloid and B‐ and T‐lymphoid lineages) or unreconstructed mice after 20 weeks, respectively (E). Black bars show the average (n ≥ 9, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test). Graphs depict the frequency of each lineage within donor‐derived cells (% Ly5.2+ cells) in the peripheral blood of recipient mice. Data are shown as means ± SD (n > 9, **P < 0.05 by the t‐test, two independent experiments) (F).

- A
A principal component analysis (PCA) using the ATAC‐seq data of HSCs in the steady state (untreated), early phase (5‐FU 6 days), or late phase (5‐FU 10 days).
- B, C
A scatter plot comparing ATAC‐seq peaks between untreated HSCs and those in the early phase (B) or between those in the early and late phases (C).
- D
ATAC peaks overlapping with H3K27ac peaks detected in HSCs in each phase or CD48+ progenitor cells in the late phase after the administration of 5‐FU were selected as cis‐regulatory regions.
- E
Scatter plots comparing peaks within cis‐regulatory regions in L−ESLAM HSCs between the early and late phases after the administration of 5‐FU.
- F
The enrichment of genes related to “accessibility‐increased regions at the late phase (Brown dot in Fig 3E)” (left) or “accessibility‐decreased regions (Green dot in Fig 3E)” (right) within genes up‐regulated in CD150+CD34− HSCs or CD150+CD34+ progenitor cells.
- G
A scatter plot comparing the peaks of cis‐regulatory regions between the steady state and early phase after the administration of 5‐FU.
- H
The enrichment of genes related to “accessibility‐increased regions from the steady state to early phase (Purple dot in Fig 3G)” in the comparison between the steady state and early phase after the administration of 5‐FU.
- I
The enrichment of genes related to “accessibility‐increased regions from the early to late phase (Brown dot in Fig 3E)” in the comparison between the early and late phases after the administration of 5‐FU.
- J
ATAC and H3K27ac peaks in the promoter and enhancer regions within the CD48 locus. ChIP‐seq data for transcription factors obtained from the public database (GSE22178) were merged.
- K
CD48 mRNA levels in HSCs after the administration of 5‐FU. Data are shown as means ± SD (n = 4, two independent experiments, N.S.; not significant by the t‐test).
- L
Average plot (top) and heatmap (bottom) of H3K27ac reads in HSCs in the early and late phases within accessibility‐decreased (left: Black dot in Fig 3H), ‐unchanged (center: Blue dot in Fig 3H), and ‐increased cis‐regulatory regions (right; Purple dot in Fig 3H) in the early phase.
- M
Average plot (top) and heatmap (bottom) of H3K27ac reads in HSCs in the early and late phases within accessibility‐decreased (left: Green dot in Fig 3E), ‐unchanged (center: Blue dot in Fig 3E), or ‐increased regions (right: Brown dot in Fig 3E) in the late phase.

The top 5 most enriched motifs within accessibility‐increased ATAC peaks (Late/Early > 2 in Fig 3C) in the late phase after the administration of 5‐FU.
Top 5 most enriched motifs within the accessibility‐increased cis‐regulatory regions in the late phase (Late/Early > 2 (Brown dot) in Fig 3E).
Genome maps showing three enhancer regions related to the Runx family. ChIP‐seq data obtained from the public database (H3K27ac in long‐term (LT)‐HSCs, short‐term (ST)‐HSC, and multi‐potent progenitors (MPPs); indicated transcription factors in HPC7) were merged.

- A
Global H3K27ac in HSCs during BM regeneration after the administration of 5‐FU. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- B
The relationship between the TCA (tricarboxylic acid cycle) cycle within mitochondrial metabolism, Acly, and histone acetylation.
- C–E
mRNA (C), protein (D), and phosphorylation levels of Ser455 (E) in Acly in HSCs after the administration of 5‐FU. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, N.S.; not significant by the t‐test).
- F
The potential for glucose uptake by HSCs obtained from untreated or 5‐FU‐treated mice was estimated using 2‐NBDG. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01, N.S., not significant by the t‐test).
- G–I
The levels of intracellular Glu (G), citrate (H), and total acetylated lysine (I) in HSCs obtained from untreated or 5‐FU‐treated mice. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01, **P < 0.05, N.S., not significant by the t‐test).
- J
ΔΨm in L−ESLAM HSCs after the administration of 5‐FU. Histograms represent the fluorescence intensity of JC‐1 Red, which is polymer‐derived fluorescence generated in response to ΔΨm. The graph depicts normalized ΔΨm by the ratio of the fluorescence intensity of JC‐1 Red to JC‐1 Green, which indicates monomer‐derived fluorescence. Data are shown as means ± SD (n ≥ 4, more than two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- K
The enrichment of the gene set “HALLMARK_OXIDATIVE_PHOSPHORYLATION” within differentially expressed genes of the steady state versus the early phase. NES and q values represent normalized enrichment scores and FDR, respectively.

- A
Schema of experimental designs and the targets of inhibitors. Serial treatments with CTPI‐2 (50 mg/kg/shot/day, i.p.), BMS303141 (100 mg/kg/shot/day, p.o.), A‐485 (20 mg/kg/shot/day, i.p.), or TV‐3664 (10 mg/kg/shot/day, p.o.) were performed 3, 4, and 5 days after the administration of 5‐FU.
- B, C
The effects of CTPI‐2 (B) or BMS303141 (BMS) (C) on H3K27ac in HSCs in the early phase after the administration of 5‐FU. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- D, E
The effects of CTPI‐2 (D), BMS303141 (BMS) (E), A‐485 (D), and TV‐3664 (E) on HSC expansion in the early phase after the administration of 5‐FU. Numbers in the dot plots represent the frequencies of the indicated fractions within total BM cells. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- F, G
Average plot (top) and heatmap (bottom) of H3K27ac reads in the cis‐regulatory regions of genes belonging to the gene set “HALLMARK_MITOTIC_SPINDLE” (F) or “HALLMARK_G2M_CHECKPOINT” (G) in HSCs in the steady state or early phase.
- H
The enrichment of the gene set “GOBP_FATTY_ACID_BIOSYNTHETIC_PROCESS” within differentially expressed genes of the steady state versus the early phase.
- I
Average plot (top) and heatmap (bottom) of H3K27ac reads in the cis‐regulatory regions of genes having the GO term “fatty acid biosynthetic process” in HSCs in the steady state or early phase.
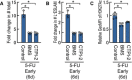
- A, B
The number or L−ESLAM phenotypic HSCs after a 4‐day culture of HSCs in the early phase after the administration of 5‐FU in the presence or absence of CTPI‐2 (50 μM), an inhibitor of mitochondrial citrate transporters, or BMS303141 (BMS; 100 μM), an inhibitor of Acly. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01 by the t‐test).
- C
H3K27ac levels after a 1‐day culture of HSCs in the early phase after the administration of 5‐FU with or without CTPI‐2 or BMS303141. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01 by the t‐test).

Expression levels of EPCR in the L−ESLAM fraction in the steady state or early phase after the administration of 5‐FU. Indicated gates within histograms were selected based on the top, middle, or lower 30% EPCR expression level in HSCs in the steady state. (n = 4, two independent experiments, *P < 0.01 by the t‐test).
According to the indicated schedule, EPCRHigh or EPCRLow L−ESLAM HSCs (Ly5.2) were transplanted into non‐irradiated Ly5.1 mice, and the chimerism of donor‐derived cells in the L−ESLAM fraction was subsequently examined after the administration of PBS or 5‐FU. Indicated gates within the dot plot for EPCR versus c‐Kit were selected based on top or lower 50% EPCR expression levels in HSCs in the steady state. The graph depicts the frequency of donor (CD45.2+)‐derived cells within the L−ESLAM fraction. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01, N.S., not significant by the t‐test).
The UMAP result of single‐cell ATAC‐seq using EPCRHigh or EPCRLow HSCs in the steady state, as defined in Fig 5B. Green and red plots represent the EPCRHigh and EPCRLow fractions, respectively. Numbers within brackets show the number used in this plot.
ATAC peaks overlapping with H3K27ac peaks detected in HSCs in each phase or CD48+ progenitor cells in the late phase were selected as cis‐regulatory regions.
A scatter plot comparing the peaks of cis‐regulatory regions between EPCR+++ and EPCR+ HSCs in the steady state.
The enrichment of genes related to “accessibility‐increased regions in the EPCR+ fraction relative to the EPCR+++ fraction (Purple dot in Fig 6E)” within genes up‐regulated in CD150+CD34− HSCs or CD150+CD34+ progenitor cells.
Fifty indicated cells were transplanted with 2 × 105 competitor cells into irradiated recipients. After 12 weeks, the chimerism of peripheral blood was analyzed. White or gray circles represent donor cell chimerism in multilineage‐reconstructed recipient mice (> 0.5% for myeloid and B‐ and T‐lymphoid lineages) or unreconstructed mice after 20 weeks, respectively. Black bars show the average (n ≥ 7, two independent experiments, *P < 0.01 by the t‐test).
Fold changes in the phenotypic HSC number after a 4‐day culture of the indicated HSC fractions. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
Venn diagrams represent the estimated frequencies of EPCR+++, EPCR++, and EPCR+ HSC‐derived cells within CD48−HSC or CD48+ progenitor cells after a 4‐day culture of HSCs derived from untreated mice. These frequencies were calculated based on the total cell number and frequency of each fraction after the culture of EPCR+++, EPCR++, and EPCR+ HSCs.
ΔΨm in CD48− HSCs and CD48+ progenitor cells after a 4‐day culture of HSCs in the steady state. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, N.S.; not significant by the t‐test).
One hundred indicated cells were transplanted with 2 × 105 competitor cells into irradiated recipients. After 20 weeks, the chimerism of peripheral blood was analyzed. White or gray circles represent donor cell chimerism in multilineage‐reconstructed recipient mice (> 0.5% for myeloid and B‐ and T‐lymphoid lineages) or unreconstructed mice after 20 weeks, respectively. Black bars show the average (n ≥ 11, two independent experiments, *P < 0.01 by the t‐test).

- A
The enrichment of the gene set “HALLMARK_G2M_CHECKPOINT” within differentially expressed genes of EPCR+++ versus EPCR+ HSCs.
- B
Total cell number after a 4‐day culture of EPCR+++, EPCR++, and EPCR+ HSCs. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- C
Cell number after a single‐cell culture for 4 days using EPCR+++, EPCR++, and EPCR+ HSCs. Data are shown as means ± SD (n = 3, three independent experiments, *P < 0.01, **P < 0.05 by the t‐test).
- D
ΔΨm in HSCs obtained from untreated mice before or after 1 day of culture. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- E
The enrichment plots of genes related to oxidative phosphorylation in the comparison of the 1‐day culture with the steady state. NES and q values represent normalized enrichment scores and FDR, respectively.
- F, G
Levels of Acly (F) and H3K27ac (G) in HSCs obtained from untreated mice before or after a 1‐day culture. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01 by the t‐test).
- H
The frequencies of the indicated fractions after a 4‐day culture of each HSC subtraction divided based on EPCR expression levels. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- I
The frequencies of the CD150+CD48− and CD48+ fractions within the lineage−EPCR+ fraction after a 4‐day culture of HSCs obtained from untreated mice with or without BMS303141 (BMS). Data are shown as means ± SD (n ≥ 6, two independent experiments, *P < 0.01, by the t‐test).
- J
Global H3K27ac after 2 days of the culture of L‐ESLAM HSCs derived from untreated mice with or without 50 μM BMS303141 (BMS). Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, **P < 0.05 by the t‐test).

- A–D
Global H3K27ac (A), Acly levels (B), acetylated lysine (C), and ΔΨm (D) in CD48− HSCs and CD48+ progenitor cells (HPCs) in the late phase after the administration of 5‐FU. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- E
The enrichment of the gene set “HALLMARK_OXIDATIVE_PHOSPHORYLATION” within differentially expressed genes between CD48− HSCs and CD48+ progenitor cells. NES and q values represent normalized enrichment scores and FDR, respectively.
- F
Average plot (top) and heatmap (bottom) of H3K27ac reads in CD48− HSCs and CD48+ HPCs in the late phase within cis‐regulatory regions related to genes with lower (left) or higher (right) expression levels in CD48+ HPCs than in CD48− HSCs.
- G
ATAC peaks of HSCs in the early or late phase, and H3K27ac peaks in CD48− HSCs or CD48+ HPCs in the late phase within the promoter and enhancer regions in the CD48 locus.
- H
CD48 mRNA levels in CD48− HSCs and CD48+ HSCs in the late phase after the administration of 5‐FU. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- I
Average plot (top) and heatmap (bottom) of H3K27ac reads in CD48− HSCs and CD48+ HPCs in the late phase within accessibility‐decreased (left: Green dot in Fig 3E), ‐unchanged (center: Blue dot in Fig 3E), or ‐increased cis‐regulatory regions (right: brown dot in Fig 3E) in HSCs in the late phase.
- J
Within accessibility‐increased cis‐regulatory regions in the late phase (brown dot in Fig 3E), 192 regions showed more than two‐fold increases in H3K27ac levels in CD48+ progenitor cells than in maintained HSCs (Venn diagrams). The GSEA plot represents the enrichment of the gene set related to these H3K27ac‐increased regions within differentially expressed genes between CD48− HSCs and CD48+ progenitor cells. NES and q values represent normalized enrichment scores and FDR, respectively.

- A, B
ΔΨm (A) and Acly levels (B) in HSCs derived from 5‐FU treated mice in the late phase before and after a 1‐day culture. Data are shown as means ± SD (n ≥ 3, two independent experiments, *P < 0.01 by the t‐test).
- C
The frequencies of the CD150+CD48− and CD48+ fractions within the lineage−EPCR+ fraction after a 4‐day culture of HSCs in the late phase with or without BMS303141 (BMS; 100 μM), SB204990 (SB; 100 μM), an alternative Acly inhibitor, or CTPI‐2 (50 μM). Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01, by the t‐test).
- D, E
Total cell number (D) or CD48+ cell number (E) after the 4‐day culture of HSCs in the late phase with or without BMS303141 (BMS), SB204990 (SB), or CTPI‐2. Numbers within the graph represent the inhibitory effects of each treatment (fold decrease by an inhibitor treatment). Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01, by the t‐test).
- F
Global H3K27ac in HSCs in the late phase before or after a 1‐day culture with or without BMS303141 (BMS), SB204990 (SB), or CTPI‐2. Data are shown as means ± SD (n ≥ 4, two independent experiments, *P < 0.01, by the t‐test).
- G
The frequencies of CD150+CD48− and CD48+ fractions within lineage−EPCR+ after HSCs in the late phase were cultured for 4 days with or without A‐485 (1 μM), an inhibitor of histone acetyltransferase. Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01 by the t‐test).
- H, I
Total cell number (H) or CD48+ cell number (I) after a 4‐day culture of HSCs in the late phase with or without A‐485. Numbers within the graph represent the inhibitory effects of each treatment (fold decrease by an inhibitor treatment). Data are shown as means ± SD (n = 4, two independent experiments, *P < 0.01, by the t‐test).
- J
The frequencies of CD150+CD48− and CD150+CD48+ fractions within lineage−EPCR+ after a serial CTPI‐2 treatment (50 mg/kg/shot/day) following the administration of 5‐FU in mice transplanted with 15,000 HSCs without irradiation. Data are shown as means ± SD (n = 4, two independent experiments, **P < 0.05 by the t‐test).
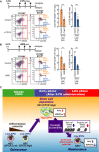
- A, B
The effects of CTPI‐2 (A) or BMS303141 (BMS) (B) on the number of HSCs and CD48+ progenitor cells in the late phase after the administration of 5‐FU. CTPI‐2 (50 mg/kg/shot/day) or BMS (100 mg/kg/shot/day) was serially treated 6, 7, and 8 days after the administration of 5‐FU. Numbers in the dot plots represent the frequency of the indicated fractions within all BM cells. Data are shown as means ± SD (n = 4, two independent experiments, **P < 0.05 by the t‐test).
- C
Proposed model (The steady state). HSCs show a quiescent state in vivo under low ΔΨm and Acly levels. EPCRlow HSCs have a chromatin accessibility pattern that enhances the differentiation potential, and EPCRHigh HSCs have a higher self‐renewal capacity. (The early phase after the administration of 5‐FU) After the administration of 5‐FU, EPCRHigh HSCs were enriched, resulting in self‐renewing divisions for HSC expansion by enhancing histone acetylation under high mitochondria‐Acly activity. (The late phase after the administration of 5‐FU) When HSCs gained an enhanced differentiation potential through the increased accessibility of progenitor cell‐related cis‐regulatory regions, the suppression of histone acetylation mediated by decreased mitochondria‐Acly activity contributed to the maintenance of stem cell phenotypes by preventing the expression of genes showing increased accessibility.
References
-
- de Bruijn M, Dzierzak E (2017) Runx transcription factors in the development and function of the definitive hematopoietic system. Blood 129: 2061–2069 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

