Partners in crime: Proteins implicated in RNA repeat expansion diseases
- PMID: 35229468
- PMCID: PMC9539487
- DOI: 10.1002/wrna.1709
Partners in crime: Proteins implicated in RNA repeat expansion diseases
Abstract
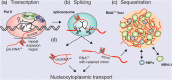
Short tandem repeats are repetitive nucleotide sequences robustly distributed in the human genome. Their expansion underlies the pathogenesis of multiple neurological disorders, including Huntington's disease, amyotrophic lateral sclerosis, and frontotemporal dementia, fragile X-associated tremor/ataxia syndrome, and myotonic dystrophies, known as repeat expansion disorders (REDs). Several molecular pathomechanisms associated with toxic RNA containing expanded repeats (RNAexp ) are shared among REDs and contribute to disease progression, however, detailed mechanistic insight into those processes is limited. To deepen our understanding of the interplay between toxic RNAexp molecules and multiple protein partners, in this review, we discuss the roles of selected RNA-binding proteins (RBPs) that interact with RNAexp and thus act as "partners in crime" in the progression of REDs. We gather current findings concerning RBPs involved at different stages of the RNAexp life cycle, such as transcription, splicing, transport, and AUG-independent translation of expanded repeats. We argue that the activity of selected RBPs can be unique or common among REDs depending on the expanded repeat type. We also present proteins that are functionally depleted due to sequestration on RNAexp within nuclear foci and those which participate in RNAexp -dependent innate immunity activation. Moreover, we discuss the utility of selected RBPs as targets in the development of therapeutic strategies. This article is categorized under: RNA Interactions with Proteins and Other Molecules > Protein-RNA Interactions: Functional Implications RNA in Disease and Development > RNA in Disease.
Keywords: RAN translation; RNA binding proteins; liquid-liquid phase separation; repeat expansion; short tandem repeats.
© 2022 The Authors. WIREs RNA published by Wiley Periodicals LLC.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



References
-
- Angelbello, A. J. , Benhamou, R. I. , Rzuczek, S. G. , Choudhary, S. , Tang, Z. , Chen, J. L. , Roy, M. , Wang, K. W. , Yildirim, I. , Jun, A. S. , Thornton, C. A. , & Disney, M. D. (2021). A small molecule that binds an RNA repeat expansion stimulates its decay via the exosome complex. Cell Chemical Biology, 28(1), 34–45.e6. 10.1016/j.chembiol.2020.10.007 - DOI - PMC - PubMed
-
- Angelbello, A. J. , Rzuczek, S. G. , Mckee, K. K. , Chen, J. L. , Olafson, H. , Cameron, M. D. , Moss, W. N. , Wang, E. T. , & Disney, M. D. (2019). Precise small‐molecule cleavage of an r(CUG) repeat expansion in a myotonic dystrophy mouse model. Proceedings of the National Academy of Sciences of the United States of America, 116(16), 7799–7804. 10.1073/pnas.1901484116 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

