Ca2+ signalling in interstitial cells of Cajal contributes to generation and maintenance of tone in mouse and monkey lower oesophageal sphincters
- PMID: 35229888
- PMCID: PMC9167266
- DOI: 10.1113/JP282570
Ca2+ signalling in interstitial cells of Cajal contributes to generation and maintenance of tone in mouse and monkey lower oesophageal sphincters
Abstract
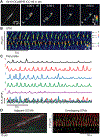
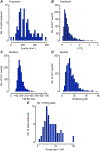
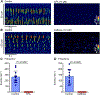
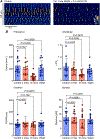
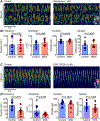
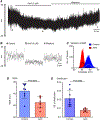
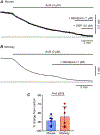
The lower oesophageal sphincter (LES) generates tone and prevents reflux of gastric contents. LES smooth muscle cells (SMCs) are relatively depolarised, facilitating activation of Cav 1.2 channels to sustain contractile tone. We hypothesised that intramuscular interstitial cells of Cajal (ICC-IM), through activation of Ca2+ -activated Cl- channels (ANO1), set membrane potentials of SMCs favourable for activation of Cav 1.2 channels. In some gastrointestinal muscles, ANO1 channels in ICC-IM are activated by Ca2+ transients, but no studies have examined Ca2+ dynamics in ICC-IM within the LES. Immunohistochemistry and qPCR were used to determine expression of key proteins and genes in ICC-IM and SMCs. These studies revealed that Ano1 and its gene product, ANO1, are expressed in c-Kit+ cells (ICC-IM) in mouse and monkey LES clasp muscles. Ca2+ signalling was imaged in situ, using mice expressing GCaMP6f specifically in ICC (Kit-KI-GCaMP6f). ICC-IM exhibited spontaneous Ca2+ transients from multiple firing sites. Ca2+ transients were abolished by cyclopiazonic acid or caffeine but were unaffected by tetracaine or nifedipine. Maintenance of Ca2+ transients depended on Ca2+ influx and store reloading, as Ca2+ transient frequency was reduced in Ca2+ free solution or by Orai antagonist. Spontaneous tone of LES muscles from mouse and monkey was reduced ∼80% either by Ani9, an ANO1 antagonist or by the Cav 1.2 channel antagonist nifedipine. Membrane hyperpolarisation occurred in the presence of Ani9. These data suggest that intracellular Ca2+ activates ANO1 channels in ICC-IM in the LES. Coupling of ICC-IM to SMCs drives depolarisation, activation of Cav 1.2 channels, Ca2+ entry and contractile tone. KEY POINTS: The lower oesophageal sphincter (LES) generates contractile tone preventing reflux of gastric contents into the oesophagus. LES smooth muscle cells (SMCs) display depolarised membrane potentials facilitating activation of L-type Ca2+ channels. Interstitial cells of Cajal (ICC) express Ca2+ -activated Cl- channels encoded by Ano1 in mouse and monkey LES. Ca2+ signalling in ICC activates ANO1 currents in ICC. ICC displayed spontaneous Ca2+ transients in mice from multiple firing sites in each cell and no entrainment of Ca2+ firing between sites or between cells. Inhibition of ANO1 channels with a specific antagonist caused hyperpolarisation of mouse LES and inhibition of tone in monkey and mouse LES muscles. Our data suggest a novel mechanism for LES tone in which Ca2+ transient activation of ANO1 channels in ICC generates depolarising inward currents that conduct to SMCs to activate L-type Ca2+ currents, Ca2+ entry and contractile tone.
Keywords: Ca2+ handling mechanisms; L-type Ca2+ channels; SIP syncytium; anoctamin-1 channels; oesophageal reflux; smooth muscle cells; swallowing reflex.
© 2022 The Authors. The Journal of Physiology © 2022 The Physiological Society.
Figures





Comment in
-
LEtS set the tone.J Physiol. 2022 Jun;600(11):2541-2542. doi: 10.1113/JP283011. Epub 2022 Apr 30. J Physiol. 2022. PMID: 35445403 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

