Feasibility of Closed-Loop Insulin Delivery with a Pregnancy-Specific Zone Model Predictive Control Algorithm
- PMID: 35230138
- PMCID: PMC9464083
- DOI: 10.1089/dia.2021.0521
Feasibility of Closed-Loop Insulin Delivery with a Pregnancy-Specific Zone Model Predictive Control Algorithm
Abstract
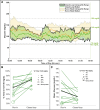
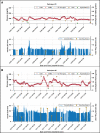
Objective: Evaluating the feasibility of closed-loop insulin delivery with a zone model predictive control (zone-MPC) algorithm designed for pregnancy complicated by type 1 diabetes (T1D). Research Design and Methods: Pregnant women with T1D from 14 to 32 weeks gestation already using continuous glucose monitor (CGM) augmented pump therapy were enrolled in a 2-day multicenter supervised outpatient study evaluating pregnancy-specific zone-MPC based closed-loop control (CLC) with the interoperable artificial pancreas system (iAPS) running on an unlocked smartphone. Meals and activities were unrestricted. The primary outcome was the CGM percentage of time between 63 and 140 mg/dL compared with participants' 1-week run-in period. Early (2-h) postprandial glucose control was also evaluated. Results: Eleven participants completed the study (age: 30.6 ± 4.1 years; gestational age: 20.7 ± 3.5 weeks; weight: 76.5 ± 15.3 kg; hemoglobin A1c: 5.6% ± 0.5% at enrollment). No serious adverse events occurred. Compared with the 1-week run-in, there was an increased percentage of time in 63-140 mg/dL during supervised CLC (CLC: 81.5%, run-in: 64%, P = 0.007) with less time >140 mg/dL (CLC: 16.5%, run-in: 30.8%, P = 0.029) and time <63 mg/dL (CLC: 2.0%, run-in:5.2%, P = 0.039). There was also less time <54 mg/dL (CLC: 0.7%, run-in:1.6%, P = 0.030) and >180 mg/dL (CLC: 4.9%, run-in: 13.1%, P = 0.032). Overnight glucose control was comparable, except for less time >250 mg/dL (CLC: 0%, run-in:3.9%, P = 0.030) and lower glucose standard deviation (CLC: 23.8 mg/dL, run-in:42.8 mg/dL, P = 0.007) during CLC. Conclusion: In this pilot study, use of the pregnancy-specific zone-MPC was feasible in pregnant women with T1D. Although the duration of our study was short and the number of participants was small, our findings add to the limited data available on the use of CLC systems during pregnancy (NCT04492566).
Keywords: Artificial pancreas; Closed loop control; Glucose control; Outpatient; Pregnancy; Type 1 diabetes.
Conflict of interest statement
C.J.L. has received research support from Insulet, Abbott Diabetes, Tandem Diabetes and Dexcom paid to her institution, and has received consulting fees from Dexcom. J.E.P. is currently an employee and shareholder of Tandem Diabetes Care, Inc., The work presented in the article was performed as part of his academic appointment at Sansum Diabetes Research Institute and is independent of his employment with Tandem Diabetes Care. G.O. receives research support from Tandem Diabetes, Insulet, Dexcom, and Abbot paid to her institution. K.C. receives research support provided to her institution from Dexcom, Abbott, Medtronic, Novonordisk, and Insulet. S.O. receives research support from Insulet, Dexcom, and Abbot paid to her institution. W.K.K. receives research funding from the NIH, DOD, AstraZeneca, Roche and Biogen all unrelated to this study. F.J.D. reports equity, licensed IP, and is a member of the Scientific Advisory Board of Mode AGC. Y.C.K. reports product support from Roche Diabetes, Dexcom, Tandem Diabetes and consulting fees from Novo Nordisk. E.D. reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this article was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. No other conflict of interest was reported.
Figures
References
-
- Maresh MJ, Holmes VA, Patterson CC, et al. : Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015;38:34–42. - PubMed
-
- Cohen O, Keidar N, Simchen M, et al. : Macrosomia in well controlled CSII treated type I diabetic pregnancy. Gynecol Endocrinol 2008;24:611–613. - PubMed
-
- Ekbom P, Damm P, Feldt-Rasmussen B, et al. : Elevated third-trimester haemoglobin A1c predicts preterm delivery in type 1 diabetes. J Diabetes Complications 2008;22:297–302. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

