CT-based Radiogenomic Analysis of Clinical Stage I Lung Adenocarcinoma with Histopathologic Features and Oncologic Outcomes
- PMID: 35230187
- PMCID: PMC9131171
- DOI: 10.1148/radiol.211582
CT-based Radiogenomic Analysis of Clinical Stage I Lung Adenocarcinoma with Histopathologic Features and Oncologic Outcomes
Abstract
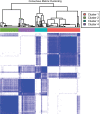
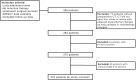
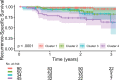
Background A preoperative predictive model is needed that can be used to identify patients with lung adenocarcinoma (LUAD) who have a higher risk of recurrence or metastasis. Purpose To investigate associations between CT-based radiomic consensus clustering of stage I LUAD and clinical-pathologic features, genomic data, and patient outcomes. Materials and Methods Patients who underwent complete surgical resection for LUAD from April 2014 to December 2017 with preoperative CT and next-generation sequencing data were retrospectively identified. Comprehensive radiomic analysis was performed on preoperative CT images; tumors were classified as solid, ground glass, or mixed. Patients were clustered into groups based on their radiomics features using consensus clustering, and clusters were compared with tumor genomic alterations, histopathologic features, and recurrence-specific survival (Kruskal-Wallis test for continuous data, χ2 or Fisher exact test for categorical data, and log-rank test for recurrence-specific survival). Cluster analysis was performed on the entire cohort and on the solid, ground-glass, and mixed lesion subgroups. Results In total, 219 patients were included in the study (median age, 68 years; interquartile range, 63-74 years; 150 [68%] women). Four radiomic clusters were identified. Cluster 1 was associated with lepidic, acinar, and papillary subtypes (76 of 90 [84%]); clusters 2 (13 of 50 [26%]) and 4 (13 of 45 [29%]) were associated with solid and micropapillary subtypes (P < .001). The EGFR alterations were highest in cluster 1 (38 of 90 [42%], P = .004). Clusters 2, 3, and 4 were associated with lymphovascular invasion (19 of 50 [38%], 14 of 34 [41%], and 28 of 45 [62%], respectively; P < .001) and tumor spread through air spaces (32 of 50 [64%], 21 of 34 [62%], and 31 of 45 [69%], respectively; P < .001). STK11 alterations (14 of 45 [31%]; P = .006), phosphoinositide 3-kinase pathway alterations (22 of 45 [49%], P < .001), and risk of recurrence (log-rank P < .001) were highest in cluster 4. Conclusion CT-based radiomic consensus clustering enabled identification of associations between radiomic features and clinicalpathologic and genomic features and outcomes in patients with clinical stage I lung adenocarcinoma. © RSNA, 2022 Online supplemental material is available for this article. See also the editorial by Nishino in this issue.
Conflict of interest statement
Figures



Comment in
-
Radiomics-based Cluster Groups to Predict Clinical-Pathologic and Genomic Characteristics of Stage I Lung Adenocarcinoma.Radiology. 2022 Jun;303(3):673-674. doi: 10.1148/radiol.213015. Epub 2022 Mar 1. Radiology. 2022. PMID: 35230188 Free PMC article. No abstract available.
References
-
- Chansky K , Sculier JP , Crowley JJ , et al . The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non–small cell lung cancer . J Thorac Oncol 2009. ; 4 ( 7 ): 792 – 801 . - PubMed
-
- Zhang J , Wu J , Tan Q , Zhu L , Gao W . Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification . J Thorac Oncol 2013. ; 8 ( 9 ): 1196 – 1202 . - PubMed
-
- Dai C , Xie H , Su H , et al . Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm . J Thorac Oncol 2017. ; 12 ( 7 ): 1052 – 1060 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

