Macrophages secrete murinoglobulin-1 and galectin-3 to regulate neutrophil degranulation after myocardial infarction
- PMID: 35230372
- PMCID: PMC8963000
- DOI: 10.1039/d1mo00519g
Macrophages secrete murinoglobulin-1 and galectin-3 to regulate neutrophil degranulation after myocardial infarction
Abstract
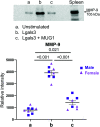
Inflammation presides early after myocardial infarction (MI) as a key event in cardiac wound healing. Ischemic cardiomyocytes secrete inflammatory cues to stimulate infiltration of leukocytes, predominantly macrophages and neutrophils. Infiltrating neutrophils degranulate to release a series of proteases including matrix metalloproteinase (MMP)-9 to break down extracellular matrix and remove necrotic myocytes to create space for the infarct scar to form. While neutrophil to macrophage communication has been explored, the reverse has been understudied. We used a proteomics approach to catalogue the macrophage secretome at MI day 1. Murinoglobulin-1 (MUG1) was the highest-ranked secreted protein (4.1-fold upregulated at MI day 1 vs. day 0 pre-MI cardiac macrophages, p = 0.004). By transcriptomics evaluation, galectin-3 (Lgals3) was 2.2-fold upregulated (p = 0.008) in MI day 1 macrophages. We explored the direct roles of MUG1 and Lgals3 on neutrophil degranulation. MUG1 blunted while Lgals3 amplified neutrophil degranulation in response to phorbol 12-myristate 13-acetate or interleukin-1β, as measured by MMP-9 secretion. Lgals3 itself also stimulated MMP-9 secretion. To determine if MUG1 regulated Lgals3, we co-stimulated neutrophils with MUG1 and Lgals3. MUG1 limited degranulation stimulated by Lgals3 by 64% (p < 0.001). In vivo, MUG1 was elevated in the infarct region at MI days 1 and 3, while Lgals3 increased at MI day 7. The ratio of MUG1 to Lgals3 positively correlated with infarct wall thickness, revealing that MUG1 attenuated infarct wall thinning. In conclusion, macrophages at MI day 1 secrete MUG1 to limit and Lgals3 to accentuate neutrophil degranulation to regulate infarct wall thinning.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution.J Mol Cell Cardiol. 2016 Nov;100:109-117. doi: 10.1016/j.yjmcc.2016.10.005. Epub 2016 Oct 13. J Mol Cell Cardiol. 2016. PMID: 27746126 Free PMC article.
-
Neutrophil proteome shifts over the myocardial infarction time continuum.Basic Res Cardiol. 2019 Aug 15;114(5):37. doi: 10.1007/s00395-019-0746-x. Basic Res Cardiol. 2019. PMID: 31418072 Free PMC article.
-
Genetic Deletion of Galectin-3 Alters the Temporal Evolution of Macrophage Infiltration and Healing Affecting the Cardiac Remodeling and Function after Myocardial Infarction in Mice.Am J Pathol. 2020 Sep;190(9):1789-1800. doi: 10.1016/j.ajpath.2020.05.010. Epub 2020 May 28. Am J Pathol. 2020. PMID: 32473918
-
Neutrophil degranulation and myocardial infarction.Cell Commun Signal. 2022 Apr 11;20(1):50. doi: 10.1186/s12964-022-00824-4. Cell Commun Signal. 2022. PMID: 35410418 Free PMC article. Review.
-
Infarct in the Heart: What's MMP-9 Got to Do with It?Biomolecules. 2021 Mar 25;11(4):491. doi: 10.3390/biom11040491. Biomolecules. 2021. PMID: 33805901 Free PMC article. Review.
Cited by
-
MMP-12 polarizes neutrophil signalome towards an apoptotic signature.J Proteomics. 2022 Jul 30;264:104636. doi: 10.1016/j.jprot.2022.104636. Epub 2022 Jun 2. J Proteomics. 2022. PMID: 35661763 Free PMC article.
-
Interaction of Iron Oxide Nanoparticles with Macrophages Is Influenced Distinctly by "Self" and "Non-Self" Biological Identities.ACS Appl Mater Interfaces. 2023 Aug 2;15(30):35906-35926. doi: 10.1021/acsami.3c05555. Epub 2023 Jul 21. ACS Appl Mater Interfaces. 2023. PMID: 37478159 Free PMC article.
-
Mapping dynamic molecular changes in hippocampal subregions after traumatic brain injury through spatial proteomics.Clin Proteomics. 2024 May 12;21(1):32. doi: 10.1186/s12014-024-09485-6. Clin Proteomics. 2024. PMID: 38735925 Free PMC article.
-
Proteomic Analysis of Human Macrophages Overexpressing Angiotensin-Converting Enzyme.Int J Mol Sci. 2024 Jun 27;25(13):7055. doi: 10.3390/ijms25137055. Int J Mol Sci. 2024. PMID: 39000163 Free PMC article.
-
Flavokavains A- and B-Free Kava Enhances Resilience against the Adverse Health Effects of Tobacco Smoke in Mice.ACS Pharmacol Transl Sci. 2024 Oct 2;7(11):3502-3517. doi: 10.1021/acsptsci.4c00415. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539272
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

