Evaluation of Budesonide-Formoterol for Maintenance and Reliever Therapy Among Patients With Poorly Controlled Asthma: A Systematic Review and Meta-analysis
- PMID: 35230437
- PMCID: PMC8889464
- DOI: 10.1001/jamanetworkopen.2022.0615
Evaluation of Budesonide-Formoterol for Maintenance and Reliever Therapy Among Patients With Poorly Controlled Asthma: A Systematic Review and Meta-analysis
Erratum in
-
Error in Figure 2B.JAMA Netw Open. 2022 May 2;5(5):e2216068. doi: 10.1001/jamanetworkopen.2022.16068. JAMA Netw Open. 2022. PMID: 35583874 Free PMC article. No abstract available.
Abstract
Importance: The Global Initiative for Asthma (GINA) recommends 2 alternative treatments for patients receiving treatment at steps 3 to 5: single inhaler combination inhaled corticosteroid-formoterol as both maintenance and reliever (SMART) or inhaled corticosteroid-long-acting β2-agonist as maintenance plus short-acting β2-agonist as reliever.
Objective: To assess whether switching to SMART is associated with longer time to first severe asthma exacerbation compared with a step up or continuation of GINA treatment step with maintenance inhaled corticosteroid-long-acting β2-agonist plus short-acting β2-agonist reliever among patients with poorly controlled asthma.
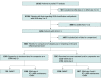
Data sources: For this systematic review and meta-analysis, the literature, internal study databases at AstraZeneca and the Medical Research Institute of New Zealand, and references from a previous systematic review and meta-analysis on SMART were searched to identify randomized clinical trials published from January 1990 to February 2018, that compared budesonide-formoterol by SMART with maintenance inhaled corticosteroid-long-acting β2-agonist plus short-acting β2-agonist reliever.
Study selection: Trials of at least 24 weeks' duration were included if they reported baseline data on GINA treatment step, asthma control status, and efficacy measures of severe exacerbations. Included patients were adults and adolescents with asthma and baseline Asthma Control Questionnaire 5-item version scores of 1.5 or higher.
Data extraction and synthesis: Patient-level data were identified by independent extraction, and analyses were performed using a fixed-effect model. Data analysis was performed from August 2018 to November 2021.
Main outcomes and measures: The primary outcome was time to first severe asthma exacerbation associated with each treatment, analyzed by Cox proportional hazards regression.
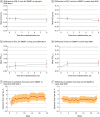
Results: Overall, 4863 patients were included (3034 [62.4%] female; mean [SD] age, 39.8 [16.3] years). Switching patients with uncontrolled asthma at GINA step 3 (n = 1950) to SMART at either step 3 or 4 was associated with a prolonged time to first severe asthma exacerbation, with a 29% reduced risk compared with stepping up to step 4 inhaled corticosteroid-long-acting β2-agonist maintenance plus short-acting β2-agonist reliever (hazard ratio, 0.71; 95% CI, 0.52-0.97). For patients with uncontrolled asthma at step 3 and step 4 (n = 2913), switching to SMART was associated with a prolonged time to first severe asthma exacerbation and a 30% reduced risk compared with remaining at the same treatment step (hazard ratio, 0.70; 95% CI, 0.58-0.85).
Conclusions and relevance: In this systematic review and meta-analysis, for patients with poorly controlled asthma, SMART was associated with longer time to first severe asthma exacerbation compared with a step up or continuation of GINA step with maintenance inhaled corticosteroid-long-acting β2-agonist plus short-acting β2-agonist reliever. These findings suggest that if an adult or adolescent receiving treatment at GINA step 3 or 4 has poorly controlled asthma, it is preferable to switch to the SMART regimen rather than to step up or continue the GINA treatment step with maintenance inhaled corticosteroid-long-acting β2-agonist plus short-acting β2-agonist reliever therapy.
Conflict of interest statement
Figures

References
-
- British Thoracic Society/SIGN . BTS/SIGN British guideline on the management of asthma. Published 2019. Accessed May 04, 2021. https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-a...
-
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Published 2018. Accessed May 04, 2021. https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf
-
- Global Initiative for Asthma (GINA). 2021 GINA report, global strategy for asthma management and prevention. Published 2021. Accessed May 04, 2021. https://ginasthma.org/gina-reports/
-
- Sobieraj DM, Weeda ER, Nguyen E, et al. . Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485-1496. doi:10.1001/jama.2018.2769 - DOI - PMC - PubMed
-
- Rogliani P, Ritondo BL, Ora J, Cazzola M, Calzetta L. SMART and as-needed therapies in mild-to-severe asthma: a network meta-analysis. Eur Respir J. 2020;56(3):2000625. - PubMed

