Asymmetric Azidation under Hydrogen Bonding Phase-Transfer Catalysis: A Combined Experimental and Computational Study
- PMID: 35230845
- PMCID: PMC8931729
- DOI: 10.1021/jacs.1c13434
Asymmetric Azidation under Hydrogen Bonding Phase-Transfer Catalysis: A Combined Experimental and Computational Study
Abstract
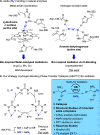
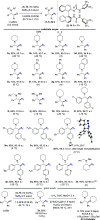
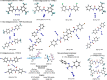
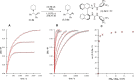
Asymmetric catalytic azidation has increased in importance to access enantioenriched nitrogen containing molecules, but methods that employ inexpensive sodium azide remain scarce. This encouraged us to undertake a detailed study on the application of hydrogen bonding phase-transfer catalysis (HB-PTC) to enantioselective azidation with sodium azide. So far, this phase-transfer manifold has been applied exclusively to insoluble metal alkali fluorides for carbon-fluorine bond formation. Herein, we disclose the asymmetric ring opening of meso aziridinium electrophiles derived from β-chloroamines with sodium azide in the presence of a chiral bisurea catalyst. The structure of novel hydrogen bonded azide complexes was analyzed computationally, in the solid state by X-ray diffraction, and in solution phase by 1H and 14N/15N NMR spectroscopy. With N-isopropylated BINAM-derived bisurea, end-on binding of azide in a tripodal fashion to all three NH bonds is energetically favorable, an arrangement reminiscent of the corresponding dynamically more rigid trifurcated hydrogen-bonded fluoride complex. Computational analysis informs that the most stable transition state leading to the major enantiomer displays attack from the hydrogen-bonded end of the azide anion. All three H-bonds are retained in the transition state; however, as seen in asymmetric HB-PTC fluorination, the H-bond between the nucleophile and the monodentate urea lengthens most noticeably along the reaction coordinate. Kinetic studies corroborate with the turnover rate limiting event resulting in a chiral ion pair containing an aziridinium cation and a catalyst-bound azide anion, along with catalyst inhibition incurred by accumulation of NaCl. This study demonstrates that HB-PTC can serve as an activation mode for inorganic salts other than metal alkali fluorides for applications in asymmetric synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Hydrogen Bonding Phase-Transfer Catalysis with Alkali Metal Fluorides and Beyond.J Am Chem Soc. 2022 Mar 30;144(12):5200-5213. doi: 10.1021/jacs.2c00190. Epub 2022 Mar 16. J Am Chem Soc. 2022. PMID: 35294171 Free PMC article.
-
Impact of Multiple Hydrogen Bonds with Fluoride on Catalysis: Insight from NMR Spectroscopy.J Am Chem Soc. 2020 Nov 18;142(46):19731-19744. doi: 10.1021/jacs.0c09832. Epub 2020 Nov 9. J Am Chem Soc. 2020. PMID: 33166450 Free PMC article.
-
Cooperative Asymmetric Cation-Binding Catalysis.Acc Chem Res. 2021 Dec 7;54(23):4319-4333. doi: 10.1021/acs.accounts.1c00400. Epub 2021 Nov 16. Acc Chem Res. 2021. PMID: 34784182
-
Multigram synthesis of N-alkyl bis-ureas for asymmetric hydrogen bonding phase-transfer catalysis.Nat Protoc. 2021 Dec;16(12):5559-5591. doi: 10.1038/s41596-021-00625-y. Epub 2021 Nov 10. Nat Protoc. 2021. PMID: 34759385 Review.
-
Harnessing Ionic Interactions and Hydrogen Bonding for Nucleophilic Fluorination.Molecules. 2020 Feb 7;25(3):721. doi: 10.3390/molecules25030721. Molecules. 2020. PMID: 32046021 Free PMC article. Review.
Cited by
-
Organocatalytic asymmetric azidation of sulfoxonium ylides: mild synthesis of enantioenriched α-azido ketones bearing a labile tertiary stereocenter.Chem Sci. 2022 Sep 15;13(39):11648-11655. doi: 10.1039/d2sc03552a. eCollection 2022 Oct 12. Chem Sci. 2022. PMID: 36320381 Free PMC article.
-
Origins of High-Activity Cage-Catalyzed Michael Addition.J Am Chem Soc. 2024 Jul 17;146(28):19317-19326. doi: 10.1021/jacs.4c05160. Epub 2024 Jul 8. J Am Chem Soc. 2024. PMID: 38976816 Free PMC article.
-
Asymmetric phase-transfer catalysis.Nat Rev Chem. 2024 Nov;8(11):851-869. doi: 10.1038/s41570-024-00642-x. Epub 2024 Oct 9. Nat Rev Chem. 2024. PMID: 39385042 Review.
-
Molecular Understanding and Practical In Silico Catalyst Design in Computational Organocatalysis and Phase Transfer Catalysis-Challenges and Opportunities.Molecules. 2023 Feb 10;28(4):1715. doi: 10.3390/molecules28041715. Molecules. 2023. PMID: 36838703 Free PMC article. Review.
-
Synthesis and Cycloaddition Reactions of 1-Azido-1,1,2,2-tetrafluoroethane.J Org Chem. 2023 Nov 3;88(21):14969-14977. doi: 10.1021/acs.joc.3c01346. Epub 2023 Oct 20. J Org Chem. 2023. PMID: 37862453 Free PMC article.
References
-
- Griess P. On a New Class of Compounds in Which Nitrogen Is Substituted for Hydrogen. Proc. R. Soc. London 1864, 13, 375–384. 10.1098/rspl.1863.0082. - DOI
- Curtius T. Ueber Stickstoffwasserstoffsäure (Azoimid) N3H. Berichte der Dtsch. Chem. Gesellschaft 1890, 23 (2), 3023–3033. 10.1002/cber.189002302232. - DOI
- Curtius T. Hydrazide Und Azide Organischer Säuren I. Abhandlung. J. für Prakt. Chemie 1894, 50 (1), 275–294. 10.1002/prac.18940500125. - DOI
- Tiemann F. Ueber Die Einwirkung von Benzolsulfonsäurechlorid Auf Amidoxime. Berichte der Dtsch. Chem. Gesellschaft 1891, 24 (2), 4162–4167. 10.1002/cber.189102402316. - DOI
-
- The Azido Group (1971); Patai S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1971.
-
-
For a recent review:
- Ding P.-G.; Hu X.-S.; Zhou F.; Zhou J. Catalytic Enantioselective Synthesis of α-Chiral Azides. Org. Chem. Front. 2018, 5 (9), 1542–1559. 10.1039/C8QO00138C. - DOI
-
Selected examples:
- Nugent W. A. Chiral Lewis Acid Catalysis. Enantioselective Addition of Azide to Meso Epoxides. J. Am. Chem. Soc. 1992, 114 (7), 2768–2769. 10.1021/ja00033a090. - DOI
- Martínez L. E.; Leighton J. L.; Carsten D. H.; Jacobsen E. N. Highly Enantioselective Ring Opening of Epoxides Catalyzed by (Salen)Cr(III) Complexes. J. Am. Chem. Soc. 1995, 117 (21), 5897–5898. 10.1021/ja00126a048. - DOI
- Hansen K. B.; Leighton J. L.; Jacobsen E. N. On the Mechanism of Asymmetric Nucleophilic Ring-Opening of Epoxides Catalyzed by (Salen)Cr(III) Complexes. J. Am. Chem. Soc. 1996, 118 (44), 10924–10925. 10.1021/ja962600x. - DOI
- Schaus S. E.; Larrow J. F.; Jacobsen E. N. Practical Synthesis of Enantiopure Cyclic 1,2-Amino Alcohols via Catalytic Asymmetric Ring Opening of Meso Epoxides. J. Org. Chem. 1997, 62 (12), 4197–4199. 10.1021/jo970146p. - DOI
- Konsler R. G.; Karl J.; Jacobsen E. N. Cooperative Asymmetric Catalysis with Dimeric Salen Complexes. J. Am. Chem. Soc. 1998, 120 (41), 10780–10781. 10.1021/ja982683c. - DOI
- Annis D. A.; Helluin O.; Jacobsen E. N. Stereochemistry as a Diversity Element: Solid-Phase Synthesis of Cyclic RGD Peptide Derivatives by Asymmetric Catalysis. Angew. Chemie Int. Ed. 1998, 37 (13–14), 1907–1909. 10.1002/(SICI)1521-3773(19980803)37:13/14<1907::AID-ANIE1907>3.0.CO;2-A. - DOI
- Myers J. K.; Jacobsen E. N. Asymmetric Synthesis of β-Amino Acid Derivatives via Catalytic Conjugate Addition of Hydrazoic Acid to Unsaturated Imides. J. Am. Chem. Soc. 1999, 121 (38), 8959–8960. 10.1021/ja991621z. - DOI
- Bellavista T.; Meninno S.; Lattanzi A.; Della Sala G. Asymmetric Hydroazidation of Nitroalkenes Promoted by a Secondary Amine-Thiourea Catalyst. Adv. Synth. Catal. 2015, 357 (14–15), 3365–3373. 10.1002/adsc.201500403. - DOI
- Nielsen M.; Zhuang W.; Jørgensen K. A. Asymmetric Conjugate Addition of Azide to α,β-Unsaturated Nitro Compounds Catalyzed by Cinchona Alkaloids. Tetrahedron 2007, 63 (26), 5849–5854. 10.1016/j.tet.2007.02.047. - DOI
- Rowland E. B.; Rowland G. B.; Rivera-Otero E.; Antilla J. C. Brønsted Acid-Catalyzed Desymmetrization of Meso-Aziridines. J. Am. Chem. Soc. 2007, 129 (40), 12084–12085. 10.1021/ja0751779. - DOI - PubMed
- Guerin D. J.; Miller S. J. Asymmetric Azidation–Cycloaddition with Open-Chain Peptide-Based Catalysts. A Sequential Enantioselective Route to Triazoles. J. Am. Chem. Soc. 2002, 124 (10), 2134–2136. 10.1021/ja0177814. - DOI - PubMed
- Horstmann T. E.; Guerin D. J.; Miller S. J. Asymmetric Conjugate Addition of Azide to α,β-Unsaturated Carbonyl Compounds Catalyzed by Simple Peptides. Angew. Chemie Int. Ed. 2000, 39 (20), 3635–3638. 10.1002/1521-3773(20001016)39:20<3635::AID-ANIE3635>3.0.CO;2-Y. - DOI - PubMed
- Tiffner M.; Stockhammer L.; Schörgenhumer J.; Röser K.; Waser M. Towards an Asymmetric Organocatalytic α-Azidation of β-Ketoesters. Molecules 2018, 23 (5), 1142.10.3390/molecules23051142. - DOI - PMC - PubMed
- Humbrías-Martín J.; Pérez-Aguilar M. C.; Mas-Ballesté R.; Dentoni Litta A.; Lattanzi A.; Della Sala G.; Fernández-Salas J. A.; Alemán J. Enantioselective Conjugate Azidation of α,β-Unsaturated Ketones under Bifunctional Organocatalysis by Direct Activation of TMSN3. Adv. Synth. Catal. 2019, 361 (20), 4790–4796. 10.1002/adsc.201900831. - DOI
- Liang Y.; Zhao X. Enantioselective Construction of Chiral Sulfides via Catalytic Electrophilic Azidothiolation and Oxythiolation of N -Allyl Sulfonamides. ACS Catal. 2019, 9 (8), 6896–6902. 10.1021/acscatal.9b01900. - DOI
-
-
- Zhdankin V. V.; Krasutsky A. P.; Kuehl C. J.; Simonsen A. J.; Woodward J. K.; Mismash B.; Bolz J. T. Preparation, X-Ray Crystal Structure, and Chemistry of Stable Azidoiodinanes-Derivatives of Benziodoxole. J. Am. Chem. Soc. 1996, 118 (22), 5192–5197. 10.1021/ja954119x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

