Upregulation of antimicrobial peptide expression in slc26a3-/- mice with colonic dysbiosis and barrier defect
- PMID: 35230892
- PMCID: PMC8890434
- DOI: 10.1080/19490976.2022.2041943
Upregulation of antimicrobial peptide expression in slc26a3-/- mice with colonic dysbiosis and barrier defect
Abstract
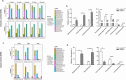
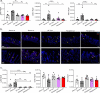
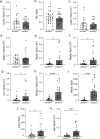
Genetic defects in SLC26A3 (DRA), an intestinal Cl-/HCO3- exchanger, result in congenital chloride diarrhea (CLD), marked by lifelong acidic diarrhea and a high risk of inflammatory bowel disease. Slc26a3-/- mice serve as a model to understand the pathophysiology of CLD and search for treatment options. This study investigates the microbiota changes in slc26a3-/- colon, the genotype-related causes for the observed microbiota alterations, its inflammatory potential, as well as the corresponding host responses. The luminal and the mucosa-adherent cecal and colonic microbiota of cohoused slc26a3-/- and wt littermates were analyzed by 16S rRNA gene sequencing. Fecal microbiota transfer from cohoused slc26a3-/- and wt littermates to germ-free wt mice was performed to analyze the stability and the inflammatory potential of the communities.The cecal and colonic luminal and mucosa-adherent microbiota of slc26a3-/- mice was abnormal from an early age, with a loss of diversity, of short-chain fatty acid producers, and an increase of pathobionts. The transfer of slc26a3-/- microbiota did not result in intestinal inflammation and the microbial diversity in the recipient mice normalized over time. A strong increase in the expression of Il22, Reg3β/γ, Relmβ, and other proteins with antimicrobial functions was observed in slc26a3-/- colon from juvenile age, while the mucosal and systemic inflammatory signature was surprisingly mild. The dysbiotic microbiota, low mucosal pH, and mucus barrier defect in slc26a3-/- colon are accompanied by a stark upregulation of the expression of a panel of antimicrobial proteins. This may explain the low inflammatory burden in the gut of these mice.
Keywords: Anion exchange; antimicrobial peptides; gut dysbiosis; inflammatory bowel disease; intestinal electrolyte transport.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Camarillo GF, Goyon EI, Zuñiga RB, Salas LAS, Escárcega AEP, Yamamoto-Furusho JK. Gene expression profiling of mediators associated with the inflammatory pathways in the intestinal tissue from patients with ulcerative colitis. Mediators Inflamm. 2020;2020:9238970. doi: 10.1155/2020/9238970. - DOI - PMC - PubMed
-
- Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol Liver Physiol. 1998;275(6):G1445–1453. doi: 10.1152/ajpgi.1998.275.6.G1445. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
