Intact mitochondrial substrate efflux is essential for prevention of tubular injury in a sex-dependent manner
- PMID: 35230975
- PMCID: PMC9057616
- DOI: 10.1172/jci.insight.150696
Intact mitochondrial substrate efflux is essential for prevention of tubular injury in a sex-dependent manner
Abstract
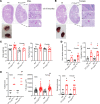
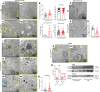
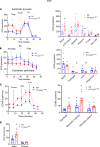
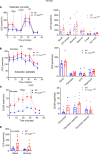
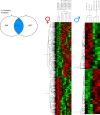
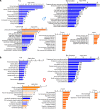
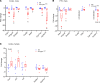
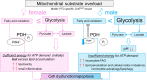
The importance of healthy mitochondrial function is implicated in the prevention of chronic kidney disease (CKD) and diabetic kidney disease (DKD). Sex differences also play important roles in DKD. Our previous studies revealed that mitochondrial substrate overload (modeled by homozygous deletion of carnitine acetyl-transferase [CrAT]) in proximal tubules causes renal injury. Here, we demonstrate the importance of intact mitochondrial substrate efflux by titrating the amount of overload through the generation of a heterozygous CrAT-KO model (PT-CrATHET mouse). Intriguingly, these animals developed renal injury similarly to their homozygous counterparts. Mitochondria were structurally and functionally impaired in both sexes. Transcriptomic analyses, however, revealed striking sex differences. Male mice shut down fatty acid oxidation and several other metabolism-related pathways. Female mice had a significantly weaker transcriptional response in metabolism, but activation of inflammatory pathways was prominent. Proximal tubular cells from PT-CrATHET mice of both sexes exhibited a shift toward a more glycolytic phenotype, but female mice were still able to oxidize fatty acid-based substrates. Our results demonstrate that maintaining mitochondrial substrate metabolism balance is crucial to satisfying proximal tubular energy demand. Our findings have potentially broad implications, as both the glycolytic shift and the sexual dimorphisms discovered herein offer potentially new modalities for future interventions for treating kidney disease.
Keywords: Chronic kidney disease; Fatty acid oxidation; Metabolism; Mitochondria; Nephrology.
Conflict of interest statement
Figures


Similar articles
-
Proximal Tubular Cell-Specific Ablation of Carnitine Acetyltransferase Causes Tubular Disease and Secondary Glomerulosclerosis.Diabetes. 2019 Apr;68(4):819-831. doi: 10.2337/db18-0090. Epub 2019 Feb 6. Diabetes. 2019. PMID: 30728184 Free PMC article.
-
The Mitochondria-Targeted Metabolic Tubular Injury in Diabetic Kidney Disease.Cell Physiol Biochem. 2019;52(2):156-171. doi: 10.33594/000000011. Epub 2019 Feb 28. Cell Physiol Biochem. 2019. PMID: 30816665 Clinical Trial.
-
CD36 deletion ameliorates diabetic kidney disease by restoring fatty acid oxidation and improving mitochondrial function.Ren Fail. 2023;45(2):2292753. doi: 10.1080/0886022X.2023.2292753. Epub 2023 Dec 14. Ren Fail. 2023. PMID: 38097943 Free PMC article.
-
Mitochondria in Diabetic Kidney Disease.Cells. 2021 Oct 29;10(11):2945. doi: 10.3390/cells10112945. Cells. 2021. PMID: 34831168 Free PMC article. Review.
-
Mitochondrial dysfunction in diabetic tubulopathy.Metabolism. 2022 Jun;131:155195. doi: 10.1016/j.metabol.2022.155195. Epub 2022 Mar 28. Metabolism. 2022. PMID: 35358497 Review.
Cited by
-
Lysosomal glucose sensing and glycophagy in metabolism.Trends Endocrinol Metab. 2023 Nov;34(11):764-777. doi: 10.1016/j.tem.2023.07.008. Epub 2023 Aug 24. Trends Endocrinol Metab. 2023. PMID: 37633800 Free PMC article. Review.
-
Redox regulation in diabetic kidney disease.Am J Physiol Renal Physiol. 2023 Aug 1;325(2):F135-F149. doi: 10.1152/ajprenal.00047.2023. Epub 2023 Jun 1. Am J Physiol Renal Physiol. 2023. PMID: 37262088 Free PMC article. Review.
-
An update on the role of sex hormones in the function of the cardiorenal mitochondria.Biochem Soc Trans. 2024 Dec 19;52(6):2307-2319. doi: 10.1042/BST20240046. Biochem Soc Trans. 2024. PMID: 39601292 Review.
-
Loss of long-chain acyl-CoA dehydrogenase protects against acute kidney injury.JCI Insight. 2025 Feb 11;10(6):e186073. doi: 10.1172/jci.insight.186073. JCI Insight. 2025. PMID: 39932791 Free PMC article.
-
Mitochondrial DNA variants in the pathogenesis and metabolic alterations of diabetes mellitus.Mol Genet Metab Rep. 2024 Dec 28;42:101183. doi: 10.1016/j.ymgmr.2024.101183. eCollection 2025 Mar. Mol Genet Metab Rep. 2024. PMID: 39835172 Free PMC article. Review.
References
-
- Sharma K. Mitochondrial dysfunction in the diabetic kidney. Adv Exp Med Biol. 2017;982:553–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

