Performance evaluation of novel fluorescent-based lateral flow immunoassay (LFIA) for rapid detection and quantification of total anti-SARS-CoV-2 S-RBD binding antibodies in infected individuals
- PMID: 35231609
- PMCID: PMC8882034
- DOI: 10.1016/j.ijid.2022.02.052
Performance evaluation of novel fluorescent-based lateral flow immunoassay (LFIA) for rapid detection and quantification of total anti-SARS-CoV-2 S-RBD binding antibodies in infected individuals
Abstract
Background: A vast majority of the commercially available lateral flow immunoassay (LFIA) is used to detect SARS-CoV-2 antibodies qualitatively. Recently, a novel fluorescence-based lateral flow immunoassay (LFIA) test was developed for quantitative measurement of the total binding antibody units (BAUs) (BAU/mL) against SARS-CoV-2 spike protein receptor-binding domain (S-RBD).
Aim: This study aimed to evaluate the performance of the fluorescence LFIA FinecareTM 2019-nCoV S-RBD test along with its reader (Model No.: FS-113).
Methods: Plasma from 150 reverse trancriptase-PCR (RT-PCR)-confirmed positive individuals and 100 prepandemic samples were tested by FincareTM to access sensitivity and specificity. For qualitative and quantitative validation of the FinCareTM measurements, BAU/mL results of FinCareTM were compared with results of 2 reference assays: the surrogate virus-neutralizing test (sVNT, GenScript Biotech, USA) and the VIDAS®3 automated assay (BioMérieux, France).
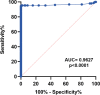
Results: FinecareTM showed 92% sensitivity and 100% specificity compared with PCR. Cohen's Kappa statistic denoted moderate and excellent agreement with sVNT and VIDAS®3, with values being 0.557 (95% CI: 0.32-0.78) and 0.731 (95% CI: 0.51-0.95), respectively. A strong correlation was observed between FinecareTM/sVNT (r = 0.7, p < 0.0001) and FinecareTM/VIDAS®3 (r = 0.8, p < 0.0001).
Conclusion: FinecareTM is a reliable assay and can be used as a surrogate to assess binding and neutralizing antibody response after infection or vaccination, particularly in none or small laboratory settings.
Keywords: COVID-19; Fluorescence immunoassay; Lateral Flow Assay; SARS-CoV-2; Serology.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest GKN would like to declare that all test kits used in this study were provided as in-kind support for his laboratory to test seroprevalence of anti-SARS-CoV-2 and antibody response among vaccinated and infected individuals in Qatar.
Figures

References
-
- Al-Thani MH, Farag E, Bertollini R, Al Romaihi HE, Abdeen S, Abdelkarim A, et al. Seroprevalence of SARS-CoV-2 infection in the craft and manual worker population of Qatar. medRxiv. 2020 doi: 10.1101/2020.11.24.20237719. - DOI
-
- Ben-David AJESwA. Comparison of classification accuracy using Cohen's Weighted Kappa. Expert Systems with Applications. 2008;34(2):825–832.
-
- Dortet L, Ronat J-B, Vauloup-Fellous C, Langendorf C, Mendels D-A, Emeraud C, et al. Evaluating 10 commercially available SARS-CoV-2 rapid serological tests by use of the STARD (Standards for Reporting of Diagnostic Accuracy Studies) method. Journal of clinical microbiology. 2021;59(2):e02342. -20. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

