Pancytopenia in a patient treated with fusidic acid and niraparib: a case report
- PMID: 35232830
- PMCID: PMC10447960
- DOI: 10.1136/ejhpharm-2021-002819
Pancytopenia in a patient treated with fusidic acid and niraparib: a case report
Abstract
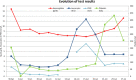
Fusidic acid is an antibiotic used in the treatment of staphylococcal infections. Niraparib is an anticancer drug indicated for the treatment of advanced ovarian cancer. The interaction between these two drugs has not been studied and is not referenced in drug databases. We present the case of a patient with pancytopenia who had been treated with fusidic acid and niraparib. No other treatment was taken by this patient. According to the literature, both substances can cause haematological toxicity. It seems unlikely that this is due to niraparib alone because it had been well tolerated by the patient for over a year before the pancytopenia was diagnosed. It was also perfectly well tolerated when it was reintroduced. We cannot determine whether this pancytopenia is due to fusidic acid alone or to a drug interaction between the two treatments. We therefore recommend caution in patients treated with this combination.
Keywords: breast neoplasms; case reports; drug incompatibility; medical oncology; pharmacy service, hospital.
© European Association of Hospital Pharmacists 2023. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Résumé des caractéristiques du produit - Acide fusidique. Available: http://agence-prd.ansm.sante.fr/php/ecodex/rcp/R0207358.htm
-
- Résumé des caractéristiques du produit - Niraparib. Available: https://ec.europa.eu/health/documents/community-register/2017/2017111613...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources