Evaluation of SARS-CoV-2 rapid antigen diagnostic tests for saliva samples
- PMID: 35233472
- PMCID: PMC8860750
- DOI: 10.1016/j.heliyon.2022.e08998
Evaluation of SARS-CoV-2 rapid antigen diagnostic tests for saliva samples
Abstract
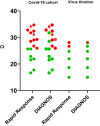
Using saliva samples would facilitate sample collection, diagnostic feasibility, and mass screening of SARS-CoV-2. We tested two rapid antigen (RAD) immunochromatographic tests designed for detection of SARS-CoV-2 in saliva: Rapid Response™ COVID-19 Antigen Rapid Test Cassette for oral fluids and DIAGNOS™ COVID-19 Antigen Saliva Test. Evaluation of detection limit was performed with purified SARS-CoV-2 nucleocapsid protein and live SARS-CoV-2 virus. Sensitivity and specificity were further evaluated with reverse transcription quantitative PCR (RT-qPCR) positive and negative saliva samples from hospitalized individuals with COVID-19 (n = 39) and healthcare workers (n = 20). DIAGNOS showed higher sensitivity than Rapid Response for both nucleocapsid protein and live virus. The limit of detection of the saliva test from DIAGNOS was further comparable with the Abbott Panbio™ COVID-19 Ag Rapid Test designed for nasopharyngeal samples. DIAGNOS and Rapid Response detected nine (50.0%) and seven (38.9%), respectively, of the 18 RT-qPCR positive saliva samples. All RT-qPCR negative saliva (n = 41) were negative with both tests. Only one of the RT-qPCR positive saliva samples contained infectious virus as determined by cell culture and was also positive using the saliva RADs. The results show that the DIAGNOS may be an important and easy-to-use saliva RAD complement to detect SARS-CoV-2 positive individuals, but validation with a larger sample set is warranted.
Keywords: Infectivity; Rapid antigen diagnostic test; SARS-CoV-2; Saliva.
© 2022 The Author(s).
Conflict of interest statement
The authors declare the following conflict of interests: Henrik Olsson and Mikael Jämtberg are Co-chief executive officers at Noviral Sweden AB, which is a distributor of the Rapid Response™ COVID-19 Antigen Rapid Test Cassette and DIAGNOS™ COVID-19 Antigen Saliva Test.
Figures
References
-
- Guglielmi G. The explosion of new coronavirus tests that could help to end the pandemic. Nature. 2020;583:506–509. - PubMed
-
- Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Muhlemann B., Zuchowski M., Jo W.K., Tscheak P., Moncke-Buchner E., Muller M.A., Krumbholz A., Drexler J.F., Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microb. 2021 - PMC - PubMed
-
- Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., Tissot-Dupont H., Million M., Drancourt M., Raoult D., Fournier P.E. Evaluation of the Panbio Covid-19 rapid antigen detection test device for the screening of patients with Covid-19. J. Clin. Microbiol. 2021;59 20. - PMC - PubMed
-
- Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Phillips S., Hooft L., Leeflang M.M., Spijker R., Van den Bruel A., Cochrane C.-D.T.A.G. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020;8 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

