SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity
- PMID: 35233544
- PMCID: PMC8761540
- DOI: 10.1016/j.xcrm.2022.100510
SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity
Abstract
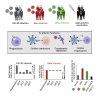
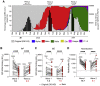
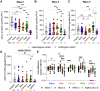
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants of concern (VOCs) exhibit escape from neutralizing antibodies, causing concern about vaccine effectiveness. However, while non-neutralizing cytotoxic functions of antibodies are associated with improved disease outcome and vaccine protection, Fc effector function escape from VOCs is poorly defined. Furthermore, whether VOCs trigger Fc functions with altered specificity, as has been reported for neutralization, is unknown. Here, we demonstrate that the Beta VOC partially evades Fc effector activity in individuals infected with the original (D614G) variant. However, not all functions are equivalently affected, suggesting differential targeting by antibodies mediating distinct Fc functions. Furthermore, Beta and Delta infection trigger responses with significantly improved Fc cross-reactivity against global VOCs compared with D614G-infected or Ad26.COV2.S-vaccinated individuals. This suggests that, as for neutralization, the infecting spike sequence affects Fc effector function. These data have important implications for vaccine strategies that incorporate VOCs, suggesting these may induce broader Fc effector responses.
Keywords: Ad26.COV2.S; Beta; Delta; Fc effector function; SARS-CoV-2; variant of concern.
© 2022.
Conflict of interest statement
P.L.M. is a member of the advisory board for Cell Reports Medicine. All other authors declare no competing interests.
Figures


References
-
- Moore P.L., Moyo-Gwete T., Hermanus T., Kgagudi P., Ayres F., Makhado Z., Sadoff J., Le Gars M., van Roey G., Crowther C., et al. Neutralizing antibodies elicited by the Ad26.COV2.S COVID-19 vaccine show reduced activity against 501Y.V2 (B.1.351), despite protection against severe disease by this variant. bioRxiv. 2021 http://biorxiv.org/content/early/2021/06/11/2021.06.09.447722.abstract
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

