mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant
- PMID: 35233550
- PMCID: PMC8784612
- DOI: 10.1016/j.xcrm.2022.100529
mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant
Abstract
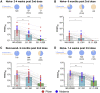
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant emerged in November 2021 and consists of several mutations within the spike. We use serum from mRNA-vaccinated individuals to measure neutralization activity against omicron in a live-virus assay. At 2-4 weeks after a primary series of vaccinations, we observe a 30-fold reduction in neutralizing activity against omicron. Six months after the initial two-vaccine doses, sera from naive vaccinated subjects show no neutralizing activity against omicron. In contrast, COVID-19-recovered individuals 6 months after receiving the primary series of vaccinations show a 22-fold reduction, with the majority of the subjects retaining neutralizing antibody responses. In naive individuals following a booster shot (third dose), we observe a 14-fold reduction in neutralizing activity against omicron, and over 90% of subjects show neutralizing activity. These findings show that a third dose is required to provide robust neutralizing antibody responses against the omicron variant.
Keywords: B.1.1.529; Omicron; Omicron variant; SARS-CoV-2; antibody; booster dose; live-virus; mRNA vaccines; neutralization assay; vaccine induced immunity.
© 2022.
Conflict of interest statement
M.S.S. serves on the advisory board for Moderna and Ocugen.
Figures
Update of
-
mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant.bioRxiv [Preprint]. 2021 Dec 22:2021.12.20.473557. doi: 10.1101/2021.12.20.473557. bioRxiv. 2021. Update in: Cell Rep Med. 2022 Jan 24;3(2):100529. doi: 10.1016/j.xcrm.2022.100529. PMID: 34981056 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

