Population Pharmacokinetic-B Cell Modeling for Ofatumumab in Patients with Relapsing Multiple Sclerosis
- PMID: 35233753
- PMCID: PMC8927028
- DOI: 10.1007/s40263-021-00895-w
Population Pharmacokinetic-B Cell Modeling for Ofatumumab in Patients with Relapsing Multiple Sclerosis
Abstract
Background: Ofatumumab, a fully human anti-CD20 monoclonal antibody indicated for the treatment of relapsing forms of multiple sclerosis (RMS), binds to a unique conformational epitope, thereby depleting B cells very efficiently and allowing subcutaneous administration at lower doses.
Objectives: The aims were to characterize the relationship between ofatumumab concentration and B cell levels, including the effect of covariates such as body weight, age, or baseline B cell count, and use simulations to confirm the chosen therapeutic dose.
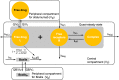
Methods: Graphical and regression analyses previously performed based on data from a dose-range finding study provided the B cell depletion target used in the present work. All available adult phase 2/3 data for ofatumumab in RMS patients were pooled to develop a population pharmacokinetics (PK)-B cell count model, using nonlinear mixed-effects modeling. The population PK-B cell model was used to simulate B cell depletion and repletion times and the effect of covariates on PK and B cell metrics, as well as the dose response across a range of subcutaneous ofatumumab monthly doses.
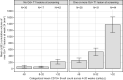
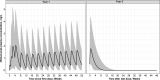
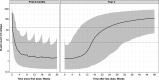
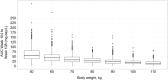
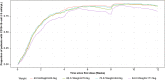
Results: The final PK-B cell model was developed using data from 1486 patients. The predetermined B cell target was best achieved and sustained with the 20-mg dose regimen, with median B cell count reaching 8 cells/µL in 11 days and negligible repletion between doses. Only weight had a significant effect on PK, which did not translate into any clinically relevant effect on B cell levels.
Conclusion: The PK-B cell modeling confirms the dose chosen for the licensed ofatumumab regimen and demonstrates no requirement for dose adjustment based on adult patient characteristics.
© 2022. The Author(s).
Conflict of interest statement
Per Soelberg Sørensen has served on scientific advisory boards for Genmab (a co-developer of ofatumumab) and GlaxoSmithKline; has served on steering committees or independent data monitoring boards in clinical trials sponsored by Genmab, GlaxoSmithKline, Merck, Novartis, and TEVA; has also served on scientific advisory boards or has received speaker honoraria for Biogen, and Celgene/BMS; has received funding of travel for these activities; and has served as editor-in-chief of the
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

