Deep learning identify retinal nerve fibre and choroid layers as markers of age-related macular degeneration in the classification of macular spectral-domain optical coherence tomography volumes
- PMID: 35233918
- PMCID: PMC9790497
- DOI: 10.1111/aos.15126
Deep learning identify retinal nerve fibre and choroid layers as markers of age-related macular degeneration in the classification of macular spectral-domain optical coherence tomography volumes
Abstract
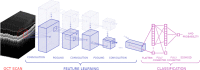
Purpose: Deep learning models excel in classifying medical image data but give little insight into the areas identified as pathology. Visualization of a deep learning model’s point of interest (POI) may reveal unexpected areas associated with diseases such as age-related macular degeneration (AMD). In this study, a deep learning model coined OptiNet was trained to identify AMD in spectral-domain optical coherence tomography (SD-OCT) macular scans and the anatomical distribution of POIs was studied.
Methods: The deep learning model OptiNet was trained and validated on two data sets. Data set no. 1 consisted of 269 AMD cases and 115 controls with one scan per person. Data set no. 2 consisted of 337 scans from 40 AMD cases (62 eyes) and 46 from both eyes of 23 controls. POIs were visualized by calculating feature dependencies across the layer hierarchy in the deep learning architecture.
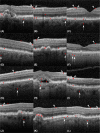
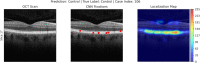
Results: The retinal nerve fibre and choroid layers were identified as POIs in 82 and 70% of cases classified as AMD by OptiNet respectively. Retinal pigment epithelium (98%) and drusen (97%) were the areas applied most frequently. OptiNet obtained area under the receiver operator curves of ≥99.7%.
Conclusion: POIs applied by the deep learning model OptiNet indicates alterations in the SD-OCT imaging regions that correspond to the retinal nerve fibre and choroid layers. If this finding represents a tissue change in macular tissue with AMD remains to be investigated, and future studies should investigate the role of the neuroretina and choroid in AMD development.
Keywords: age-related macular degeneration; convolutional neural network; deep learning; explainable artificial intelligence; spectral-domain optical coherence tomography; visualization.
Figures



References
-
- Apostolopoulos S, Ciller C, De Zanet SI, Wolf S & Sznitman R (2016): RetiNet: Automatic AMD identification in OCT volumetric data. arXiv preprint: arXiv:1610.03628.
-
- Bany M & Yeasin M (2021): Eigen‐CAM: visual explanations for deep convolutional neural networks. SN Comput Sci 2: 47.
-
- Chen TC, Cense B, Pierce MC et al. (2005): Spectral domain optical coherence tomography: ultra‐high speed, ultra‐high resolution ophthalmic imaging. Arch Ophthalmol 123: 1715–1720. - PubMed
-
- Cho J, Lee K, Shin E, Choy G & Do S (2015): How much data is needed to train a medical image deep learning system to achieve necessary high accuracy?. arXiv preprint: arXiv:1511.06348.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

