Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis
- PMID: 35234334
- PMCID: PMC9437140
- DOI: 10.1002/alz.12610
Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis
Abstract
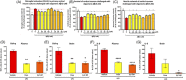
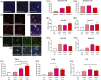
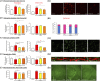
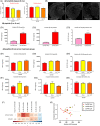
Objective: Evaluating the efficacy of 3,6'-dithioPomalidomide in 5xFAD Alzheimer's disease (AD) mice to test the hypothesis that neuroinflammation is directly involved in the development of synaptic/neuronal loss and cognitive decline.
Background: Amyloid-β (Aβ) or tau-focused clinical trials have proved unsuccessful in mitigating AD-associated cognitive impairment. Identification of new drug targets is needed. Neuroinflammation is a therapeutic target in neurodegenerative disorders, and TNF-α a pivotal neuroinflammatory driver.
New hypothesis: AD-associated chronic neuroinflammation directly drives progressive synaptic/neuronal loss and cognitive decline. Pharmacologically mitigating microglial/astrocyte activation without altering Aβ generation will define the role of neuroinflammation in AD progression.
Major challenges: Difficulty of TNF-α-lowering compounds reaching brain, and identification of a therapeutic-time window to preserve the beneficial role of neuroinflammatory processes.
Linkage to other major theories: Microglia/astroglia are heavily implicated in maintenance of synaptic plasticity/function in healthy brain and are disrupted by Aβ. Mitigation of chronic gliosis can restore synaptic homeostasis/cognitive function.
Keywords: 3,6’-dithioPomalidomide; 5xFAD mice; Alzheimer pathology; TNF-α; amyloid hypothesis; microglial/astrocyte activation; neuroinflammation.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Conflict of interest statement
3,6’‐Dithiopomalidomide (3,6’‐DP) is protected under US Patent 8,927,725 to support its development for the treatment of neurodegenerative disorders. DT, WL and NHG are named inventors on this patent and have assigned all their rights to the National Institutes of Health (US Government), and hence have no ownership of 3,6’‐DP or other agents within US Patent 8,927,725. DSK is the founding scientist and CEO of Aevis Bio, Inc., and its US division AevisBio, Inc., which have a Cooperative Research and Development Agreement with the National Institute on Aging, National Institutes of Health, in relation to the evaluation of thalidomide analogs in preclinical models of neurodegenerative disorders. IH, YKK and SK are employees of Aevis Bio, Inc. All other authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

