Pathophysiology of systemic sclerosis (scleroderma)
- PMID: 35234358
- PMCID: PMC11896191
- DOI: 10.1002/kjm2.12505
Pathophysiology of systemic sclerosis (scleroderma)
Abstract
Systemic sclerosis (scleroderma) is an autoimmune-triggered chronic fibrosing disease that affects the skin and many other organs. Its pathophysiology is complex and involves an early endothelial damage, an inflammatory infiltrate and a resulting fibrotic reaction. Based on a predisposing genetic background, an altered balance of the acquired and the innate immune system leads to the release of many cytokines and chemokines as well as autoantibodies, which induce the activation of fibroblasts with the formation of myofibroblasts and the deposition of a stiff and rigid connective tissue. A curative treatment is still not available but remarkable progress has been made in the management of organ complications. In addition, several breakthroughs in the pathophysiology have led to new therapeutic concepts. Based on these, many new compounds have been developed during the last years, which target these different pathways and offer specific therapeutic approaches.
Keywords: extracellular matrix; fibroblast; fibrosis; inflammation.
© 2022 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia Ltd on behalf of Kaohsiung Medical University.
Conflict of interest statement
All authors declare no conflict of interest.
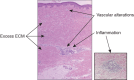
Figures


References
-
- Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al. European dermatology forum S1‐guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol. 2017;31(9):1401–24. - PubMed
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003. - PubMed
-
- Eckes B, Moinzadeh P, Sengle G, Hunzelmann N, Krieg T. Molecular and cellular basis of scleroderma. J Mol Med (Berl). 2014;92(9):913–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

