Concurrent stimulation and sensing in bi-directional brain interfaces: a multi-site translational experience
- PMID: 35234664
- PMCID: PMC9095704
- DOI: 10.1088/1741-2552/ac59a3
Concurrent stimulation and sensing in bi-directional brain interfaces: a multi-site translational experience
Abstract
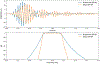
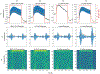
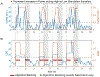
Objective. To provide a design analysis and guidance framework for the implementation of concurrent stimulation and sensing during adaptive deep brain stimulation (aDBS) with particular emphasis on artifact mitigations.Approach. We defined a general architecture of feedback-enabled devices, identified key components in the signal chain which might result in unwanted artifacts and proposed methods that might ultimately enable improved aDBS therapies. We gathered data from research subjects chronically-implanted with an investigational aDBS system, Summit RC + S, to characterize and explore artifact mitigations arising from concurrent stimulation and sensing. We then used a prototype investigational implantable device, DyNeuMo, and a bench-setup that accounts for tissue-electrode properties, to confirm our observations and verify mitigations. The strategies to reduce transient stimulation artifacts and improve performance during aDBS were confirmed in a chronic implant using updated configuration settings.Main results.We derived and validated a 'checklist' of configuration settings to improve system performance and areas for future device improvement. Key considerations for the configuration include (a) active instead of passive recharge, (b) sense-channel blanking in the amplifier, (c) high-pass filter settings, (d) tissue-electrode impedance mismatch management, (e) time-frequency trade-offs in the classifier, (f) algorithm blanking and transition rate limits. Without proper channel configuration, the aDBS algorithm was susceptible to limit-cycles of oscillating stimulation independent of physiological state. By applying the checklist, we could optimize each block's performance characteristics within the overall system. With system-level optimization, a 'fast' aDBS prototype algorithm was demonstrated to be feasible without reentrant loops, and with noise performance suitable for subcortical brain circuits.Significance. We present a framework to study sources and propose mitigations of artifacts in devices that provide chronic aDBS. This work highlights the trade-offs in performance as novel sensing devices translate to the clinic. Finding the appropriate balance of constraints is imperative for successful translation of aDBS therapies.Clinical trial:Institutional Review Board and Investigational Device Exemption numbers: NCT02649166/IRB201501021 (University of Florida), NCT04043403/IRB52548 (Stanford University), NCT03582891/IRB1824454 (University of California San Francisco). IDE #180 097.
Keywords: adaptive deep brain stimulation; algorithms; artifacts; chronic implant; closed loop; embedded; neural sensing.
Creative Commons Attribution license.
Conflict of interest statement
Conflict of interest
JA and RG have worked for Rune Labs Inc. since September 2021. SL is a scientific advisor to Rune Labs Inc. and has received reimbursement for educational activities for Medtronic. Bronte-Stewart serves on a clinical advisory board for Medtronic PLC. TD has received funding for educational activities by Medtronic PLC, has stock ownership in Bioinduction Ltd and Amber Therapeutics, has consulted with Synchron, Cortec Neuro, and Amber Therapeutics.
Figures


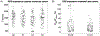

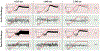

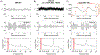
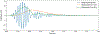
References
-
- Fountas KN and Smith JR 2007. A novel closed-loop stimulation system in the control of focal, medically refractory epilepsy Acta Neurochir. Suppl 97 357–62 - PubMed
-
- Herron J, Linde D, Chouinard T, Isaacson B, Stanslaski S, Bourget D, Adamski T and Denison T 2018. Embedding adaptive stimulation algorithms for a new implantable deep-brain stimulation research tool 2018 IEEE Biomedical Circuits and Systems Conf (Biocas) pp 1–4
