Sustained Impact of the Coronavirus Disease 2019 Pandemic on Hepatitis C Virus Treatment Initiations in the United States
- PMID: 35234860
- PMCID: PMC9129135
- DOI: 10.1093/cid/ciac175
Sustained Impact of the Coronavirus Disease 2019 Pandemic on Hepatitis C Virus Treatment Initiations in the United States
Abstract
Background: Recent reports indicated declines in hepatitis C virus (HCV) testing during the first half of 2020 in the United States due to coronavirus disease 2019 (COVID-19), but the longer-term impact on HCV testing and treatment is unclear.
Methods: We obtained monthly state-level volumes of HCV antibody, RNA and genotype testing, and HCV treatment initiation, stratified by age and gender, spanning January 2019 until December 2020 from 2 large national laboratories. We performed segmented regression analysis for each state from a mixed-effects Poisson regression model with month as the main fixed predictor and state as a random intercept.
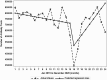
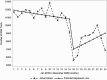
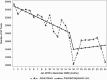
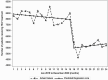
Results: During the pre-COVID-19 period (January 2019-March 2020), monthly HCV antibody and genotype tests decreased slightly whereas RNA tests and treatment initiations remained stable. Between March and April 2020, there were declines in the number of HCV antibody tests (37% reduction, P < .001), RNA tests (37.5% reduction, P < .001), genotype tests (24% reduction, P = .023), and HCV treatment initiations (31%, P < .001). Starting April 2020 through the end of 2020, there were significant increases in month-to-month HCV antibody (P < .001), RNA (P = .035), and genotype tests (P = .047), but only antibody testing rebounded to pre-COVID-19 levels. HCV treatment initiations remained low after April 2020 throughout the remainder of the year.
Conclusions: HCV testing and treatment dropped by >30% during April 2020 at the start of the COVID-19 pandemic, but although HCV testing increased again later in 2020, HCV treatment rates did not recover. Efforts should be made to link HCV-positive patients to treatment and revitalize HCV treatment engagement by healthcare providers.
Keywords: RNA; antibody; coronavirus; hepatitis C virus; testing.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures