Studies on the binding of nitrogenous bases to protoporphyrin IX iron(II) in aqueous solution at high pH values
- PMID: 35235042
- PMCID: PMC8960585
- DOI: 10.1007/s00775-022-01929-4
Studies on the binding of nitrogenous bases to protoporphyrin IX iron(II) in aqueous solution at high pH values
Abstract
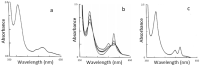
Studies are reported on the formation of low-spin six-coordinate [Fe(PPIX)L2] complexes from iron(II) protoporphyrin where L is one of a series of nitrogenous ligands (aliphatic, aromatic or heterocyclic). The bonding constants have been determined by titration of the metal complex with these ligands and are compared in relation to previous studies. The adduct formation was monitored utilising optical spectroscopy. In addition, Mӧssbauer spectroscopic experiments were conducted to monitor the electronic environment around the central iron atom in these complexes. The two complementary spectroscopic methods indicated that all nitrogen ligands formed low-spin octahedral complexes. The magnitude of the overall binding constants (β2 values) are discussed and related to (a) the pKa values of the free ligands and (b) the Mössbauer parameter ΔEQ, which represents the quadrupole splitting of the haem iron. The β2 and ΔEQ values are also discussed in terms of the structure of the ligand. Cooperative binding was observed for nearly all the ligands with Hill coefficients close to 2 for iron(II) protoporphyrin; one of these ligands displayed a much greater affinity than any we previously studied, and this was a direct consequence of the structure of the ligand. Overall conclusions on these and previous studies are drawn in terms of aliphatic ligands versus aromatic ring structures and the absence or presence of sterically hindered nitrogen atoms. The implications of the work for the greater understanding of haem proteins in general and in particular how the nitrogenous ligand binding results are relevant to and aid the understanding of the binding of inhibitor molecules to the cytochrome P450 mono-oxygenases (for therapeutic purposes) are also discussed. Changes in the electronic absorption spectra of five-coordinate [Fe(II)(PPIX)(2-MeIm)] that occurred as the temperature was lowered from room temperature to 78° K.
Keywords: Hill coefficients; Mossbauer spectroscopy; Nitrogenous ligands; Protoporphyrin IX iron(II); Stability constants.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. Amsterdam: North-Holland; 1971.
-
- Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Health Sci J. 2014;8:272–290.
-
- Turino GM. Circulation (United States) 1981;63:A253–A259.
-
- Lemberg R, Barrett J. Cytochromes. London: Academic Press; 1971.
-
- Wilson MT (1993) In Silver J (ed) Chemistry of iron. Blackie Academic and Professional, London
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

