Mitochondria in cone photoreceptors act as microlenses to enhance photon delivery and confer directional sensitivity to light
- PMID: 35235359
- PMCID: PMC8890704
- DOI: 10.1126/sciadv.abn2070
Mitochondria in cone photoreceptors act as microlenses to enhance photon delivery and confer directional sensitivity to light
Abstract
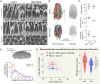
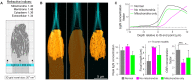
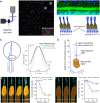
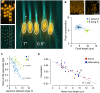
Mammalian photoreceptors aggregate numerous mitochondria, organelles chiefly for energy production, in the ellipsoid region immediately adjacent to the light-sensitive outer segment to support the high metabolic demands of phototransduction. However, these complex, lipid-rich organelles are also poised to affect light passage into the outer segment. Here, we show, via live imaging and simulations, that despite this risk of light scattering or absorption, these tightly packed mitochondria "focus" light for entry into the outer segment and that mitochondrial remodeling affects such light concentration. This "microlens"-like feature of cone mitochondria delivers light with an angular dependence akin to the Stiles-Crawford effect (SCE), providing a simple explanation for this essential visual phenomenon that improves resolution. This new insight into the optical role of mitochondria is relevant for the interpretation of clinical ophthalmological imaging, lending support for the use of SCE as an early diagnostic tool in retinal disease.
Figures


References
-
- Frazier A. E., Kiu C., Stojanovski D., Hoogenraad N. J., Ryan M. T., Mitochondrial morphology and distribution in mammalian cells. Biol. Chem. 387, 1551–1558 (2006). - PubMed
-
- Hoang Q. V., Linsenmeier R. A., Chung C. K., Curcio C. A., Photoreceptor inner segments in monkey and human retina: Mitochondrial density, optics, and regional variation. Vis. Neurosci. 19, 395–407 (2002). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

