Novel Role of Prereplication Complex Component Cell Division Cycle 6 in Retinal Neovascularization
- PMID: 35236105
- PMCID: PMC8957605
- DOI: 10.1161/ATVBAHA.121.317182
Novel Role of Prereplication Complex Component Cell Division Cycle 6 in Retinal Neovascularization
Abstract
Background: The major aim of this study is to investigate whether CDC6 (cell division cycle 6), a replication origin recognition complex component, plays a role in retinal neovascularization, and if so, to explore the underlying mechanisms.
Methods: In this study, we used a variety of approaches including cellular and moleculer biological methodologies as well as global and tissue-specific knockout mice in combination with an oxygen-induced retinopathy model to study the role of CDC6 in retinal neovascularization.
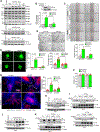
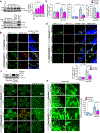
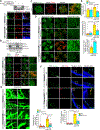
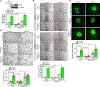
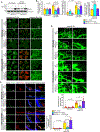
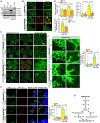
Results: VEGFA (vascular endothelial growth factor A)-induced CDC6 expression in a time-dependent manner in human retinal microvascular endothelial cells. In addition, VEGFA-induced CDC6 expression was dependent on PLCβ3 (phospholipase Cβ3)-mediated NFATc1 (nuclear factor of activated T cells c1) activation. Furthermore, while siRNA-mediated depletion of PLCβ3, NFATc1, or CDC6 levels blunted VEGFA-induced human retinal microvascular endothelial cell angiogenic events such as proliferation, migration, sprouting, and tube formation, CDC6 overexpression rescued these effects in NFATc1-deficient mouse retinal microvascular endothelial cells. In accordance with these observations, global knockdown of PLCβ3 or endothelial cell-specific deletion of NFATc1 or siRNA-mediated depletion of CDC6 levels substantially inhibited oxygen-induced retinopathy-induced retinal sprouting and neovascularization. In addition, retroviral-mediated overexpression of CDC6 rescued oxygen-induced retinopathy-induced retinal neovascularization from inhibition in PLCβ3 knockout mice and in endothelial cell-specific NFATc1-deficient mice.
Conclusions: The above observations clearly reveal that PLCβ3-mediated NFATc1 activation-dependent CDC6 expression plays a crucial role in VEGFA/oxygen-induced retinopathy-induced retinal neovascularization.
Keywords: endothelial cell; knockout mice; origin recognition complex; oxygen; retinal neovascularization.
Figures


Similar articles
-
Requirement of Site-Specific Tyrosine Phosphorylation of Cortactin in Retinal Neovascularization and Vascular Leakage.Arterioscler Thromb Vasc Biol. 2024 Feb;44(2):366-390. doi: 10.1161/ATVBAHA.123.320279. Epub 2023 Dec 21. Arterioscler Thromb Vasc Biol. 2024. PMID: 38126170 Free PMC article.
-
A new role for cofilin in retinal neovascularization.J Cell Sci. 2016 Mar 15;129(6):1234-49. doi: 10.1242/jcs.179382. Epub 2016 Feb 8. J Cell Sci. 2016. PMID: 26857814 Free PMC article.
-
PKCθ-JunB axis via upregulation of VEGFR3 expression mediates hypoxia-induced pathological retinal neovascularization.Cell Death Dis. 2020 May 7;11(5):325. doi: 10.1038/s41419-020-2522-0. Cell Death Dis. 2020. PMID: 32382040 Free PMC article.
-
Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCγ-cPLA(2) activation and retinal neovascularization.Blood. 2013 Mar 7;121(10):1911-23. doi: 10.1182/blood-2012-03-419234. Epub 2013 Jan 14. Blood. 2013. PMID: 23319572 Free PMC article.
-
[Cell biology of intraocular vascular diseases].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):923-47. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643294 Review. Japanese.
Cited by
-
Unraveling the Molecular Mechanisms of SIRT7 in Angiogenesis: Insights from Substrate Clues.Int J Mol Sci. 2024 Oct 28;25(21):11578. doi: 10.3390/ijms252111578. Int J Mol Sci. 2024. PMID: 39519130 Free PMC article. Review.
-
Requirement of Site-Specific Tyrosine Phosphorylation of Cortactin in Retinal Neovascularization and Vascular Leakage.Arterioscler Thromb Vasc Biol. 2024 Feb;44(2):366-390. doi: 10.1161/ATVBAHA.123.320279. Epub 2023 Dec 21. Arterioscler Thromb Vasc Biol. 2024. PMID: 38126170 Free PMC article.
References
-
- World report on vision. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789241516570.
-
- Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K, Reinblatt M, Steenbergen C, Harmon JW, Gerecht S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A. 2011;108:20976–20981. - PMC - PubMed
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

