Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite
- PMID: 35236743
- PMCID: PMC8896050
- DOI: 10.1136/jitc-2021-003725
Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite
Abstract
Background: Despite the great success, the therapeutic benefits of immune checkpoint inhibitors (ICIs) in cancer immunotherapy are limited by either various resistance mechanisms or ICI-associated toxic effects including gastrointestinal toxicity. Thus, novel therapeutic strategies that provide manageable side effects to existing ICIs would enhance and expand their therapeutic efficacy and application. Due to its proven role in cancer development and immune regulation, gut microbiome has gained increasing expectation as a potential armamentarium to optimize immunotherapy with ICI. However, much has to be learned to fully harness gut microbiome for clinical applicability. Here we have assessed whether microbial metabolites working at the interface between microbes and the host immune system may optimize ICI therapy.
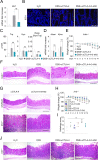
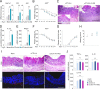
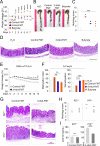
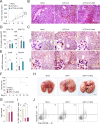
Methods: To this purpose, we have tested indole-3-carboxaldehyde (3-IAld), a microbial tryptophan catabolite known to contribute to epithelial barrier function and immune homeostasis in the gut via the aryl hydrocarbon receptor (AhR), in different murine models of ICI-induced colitis. Epithelial barrier integrity, inflammation and changes in gut microbiome composition and function were analyzed. AhR, indoleamine 2,3-dioxygenase 1, interleukin (IL)-10 and IL-22 knockout mice were used to investigate the mechanism of 3-IAld activity. The function of the microbiome changes induced by 3-IAld was evaluated on fecal microbiome transplantation (FMT). Finally, murine tumor models were used to assess the effect of 3-IAld treatment on the antitumor activity of ICI.
Results: On administration to mice with ICI-induced colitis, 3-IAld protected mice from intestinal damage via a dual action on both the host and the microbes. Indeed, paralleling the activation of the host AhR/IL-22-dependent pathway, 3-IAld also affected the composition and function of the microbiota such that FMT from 3-IAld-treated mice protected against ICI-induced colitis with the contribution of butyrate-producing bacteria. Importantly, while preventing intestinal damage, 3-IAld did not impair the antitumor activity of ICI.
Conclusions: This study provides a proof-of-concept demonstration that moving past bacterial phylogeny and focusing on bacterial metabolome may lead to a new class of discrete molecules, and that working at the interface between microbes and the host immune system may optimize ICI therapy.
Keywords: cytotoxicity; immune tolerance; immunologic; immunotherapy; inflammation; melanoma.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
