A Novel Soluble ACE2 Protein Provides Lung and Kidney Protection in Mice Susceptible to Lethal SARS-CoV-2 Infection
- PMID: 35236774
- PMCID: PMC9257820
- DOI: 10.1681/ASN.2021091209
A Novel Soluble ACE2 Protein Provides Lung and Kidney Protection in Mice Susceptible to Lethal SARS-CoV-2 Infection
Abstract
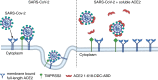
Background: Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) uses full-length angiotensin converting enzyme 2 (ACE2) as a main receptor to enter target cells. The goal of this study was to demonstrate the preclinical efficacy of a novel soluble ACE2 protein with increased duration of action and binding capacity in a lethal mouse model of COVID-19.
Methods: A human soluble ACE2 variant fused with an albumin binding domain (ABD) was linked via a dimerization motif hinge-like 4-cysteine dodecapeptide (DDC) to improve binding capacity to SARS-CoV-2. This novel soluble ACE2 protein (ACE2-1-618-DDC-ABD) was then administered intranasally and intraperitoneally to mice before intranasal inoculation of SARS-CoV-2 and then for two additional days post viral inoculation.
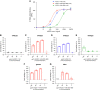
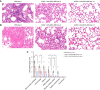
Results: Untreated animals became severely ill, and all had to be humanely euthanized by day 6 or 7 and had pulmonary alveolar hemorrhage with mononuclear infiltrates. In contrast, all but one mouse infected with a lethal dose of SARS-CoV-2 that received ACE2-1-618-DDC-ABD survived. In the animals inoculated with SARS-CoV-2 that were untreated, viral titers were high in the lungs and brain, but viral titers were absent in the kidneys. Some untreated animals, however, had variable degrees of kidney proximal tubular injury as shown by attenuation of the proximal tubular brush border and increased NGAL and TUNEL staining. Viral titers in the lung and brain were reduced or nondetectable in mice that received ACE2-1-618-DDC-ABD, and the animals developed only moderate disease as assessed by a near-normal clinical score, minimal weight loss, and improved lung and kidney injury.
Conclusions: This study demonstrates the preclinical efficacy of a novel soluble ACE2 protein, termed ACE2-1-618-DDC-ABD, in a lethal mouse model of SARS-CoV-2 infection that develops severe lung injury and variable degrees of moderate kidney proximal tubular injury.
Keywords: COVID-19; SARS-CoV-2; acute renal failure; angiotensin-converting enzyme 2; disease susceptibility; kidney disease; mice; renal protection; renin angiotensin system; virology.
Copyright © 2022 by the American Society of Nephrology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

