FSH blockade improves cognition in mice with Alzheimer's disease
- PMID: 35236988
- PMCID: PMC9940301
- DOI: 10.1038/s41586-022-04463-0
FSH blockade improves cognition in mice with Alzheimer's disease
Abstract
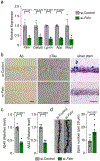
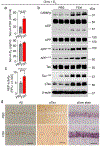
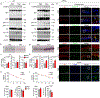
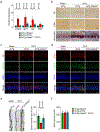
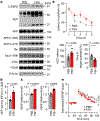
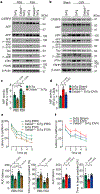
Alzheimer's disease has a higher incidence in older women, with a spike in cognitive decline that tracks with visceral adiposity, dysregulated energy homeostasis and bone loss during the menopausal transition1,2. Inhibiting the action of follicle-stimulating hormone (FSH) reduces body fat, enhances thermogenesis, increases bone mass and lowers serum cholesterol in mice3-7. Here we show that FSH acts directly on hippocampal and cortical neurons to accelerate amyloid-β and Tau deposition and impair cognition in mice displaying features of Alzheimer's disease. Blocking FSH action in these mice abrogates the Alzheimer's disease-like phenotype by inhibiting the neuronal C/EBPβ-δ-secretase pathway. These data not only suggest a causal role for rising serum FSH levels in the exaggerated Alzheimer's disease pathophysiology during menopause, but also reveal an opportunity for treating Alzheimer's disease, obesity, osteoporosis and dyslipidaemia with a single FSH-blocking agent.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









Comment in
-
Hooking FSH as a potential target for Alzheimer disease.Nat Rev Drug Discov. 2022 Apr;21(4):259. doi: 10.1038/d41573-022-00052-y. Nat Rev Drug Discov. 2022. PMID: 35277675 No abstract available.
-
FSH provides link between menopause and AD.Nat Rev Neurol. 2022 May;18(5):251. doi: 10.1038/s41582-022-00647-4. Nat Rev Neurol. 2022. PMID: 35292744 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

