Vav3-Deficient Astrocytes Enhance the Dendritic Development of Hippocampal Neurons in an Indirect Co-culture System
- PMID: 35237130
- PMCID: PMC8882586
- DOI: 10.3389/fncel.2021.817277
Vav3-Deficient Astrocytes Enhance the Dendritic Development of Hippocampal Neurons in an Indirect Co-culture System
Abstract
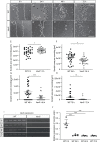
Vav proteins belong to the class of guanine nucleotide exchange factors (GEFs) that catalyze the exchange of guanosine diphosphate (GDP) by guanosine triphosphate (GTP) on their target proteins. Here, especially the members of the small GTPase family, Ras homolog family member A (RhoA), Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division control protein 42 homolog (Cdc42) can be brought into an activated state by the catalytic activity of Vav-GEFs. In the central nervous system (CNS) of rodents Vav3 shows the strongest expression pattern in comparison to Vav2 and Vav1, which is restricted to the hematopoietic system. Several studies revealed an important role of Vav3 for the elongation and branching of neurites. However, little is known about the function of Vav3 for other cell types of the CNS, like astrocytes. Therefore, the following study analyzed the effects of a Vav3 knockout on several astrocytic parameters as well as the influence of Vav3-deficient astrocytes on the dendritic development of cultured neurons. For this purpose, an indirect co-culture system of native hippocampal neurons and Vav3-deficient cortical astrocytes was used. Interestingly, neurons cultured in an indirect contact with Vav3-deficient astrocytes showed a significant increase in the dendritic complexity and length after 12 and 17 days in vitro (DIV). Furthermore, Vav3-deficient astrocytes showed an enhanced regeneration in the scratch wound heal assay as well as an altered profile of released cytokines with a complete lack of CXCL11, reduced levels of IL-6 and an increased release of CCL5. Based on these observations, we suppose that Vav3 plays an important role for the development of dendrites by regulating the expression and the release of neurotrophic factors and cytokines in astrocytes.
Keywords: CCL5; Rho-GTPases; Vav3; astrocytes; cytokines; guanine nucleotide exchange factor; hippocampal neurons; neuron-astrocyte interaction.
Copyright © 2022 Wegrzyn, Zokol and Faissner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Deletion of the Nucleotide Exchange Factor Vav3 Enhances Axonal Complexity and Synapse Formation but Tampers Activity of Hippocampal Neuronal Networks In Vitro.Int J Mol Sci. 2020 Jan 28;21(3):856. doi: 10.3390/ijms21030856. Int J Mol Sci. 2020. PMID: 32013053 Free PMC article.
-
Involvement of the guanine nucleotide exchange factor Vav3 in central nervous system development and plasticity.Biol Chem. 2017 May 1;398(5-6):663-675. doi: 10.1515/hsz-2016-0275. Biol Chem. 2017. PMID: 28214347 Review.
-
Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes.J Biol Chem. 2002 May 17;277(20):17638-48. doi: 10.1074/jbc.M111575200. Epub 2002 Mar 7. J Biol Chem. 2002. PMID: 11884391
-
The guanine nucleotide exchange factor Vav3 modulates oligodendrocyte precursor differentiation and supports remyelination in white matter lesions.Glia. 2019 Feb;67(2):376-392. doi: 10.1002/glia.23548. Epub 2018 Nov 18. Glia. 2019. PMID: 30450647
-
VAV3 in human cancers: Mechanism and clinical implication.Pathol Res Pract. 2023 Aug;248:154681. doi: 10.1016/j.prp.2023.154681. Epub 2023 Jul 13. Pathol Res Pract. 2023. PMID: 37467637 Review.
Cited by
-
mosGraphFlow: a novel integrative graph AI model mining disease targets from multi-omic data.bioRxiv [Preprint]. 2024 Sep 3:2024.08.01.606219. doi: 10.1101/2024.08.01.606219. bioRxiv. 2024. PMID: 39282361 Free PMC article. Preprint.
-
The guanine nucleotide exchange factor Vav3 intervenes in the migration pathway of oligodendrocyte precursor cells on tenascin-C.Front Cell Dev Biol. 2022 Nov 30;10:1042403. doi: 10.3389/fcell.2022.1042403. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531963 Free PMC article.
-
1-L Transcription in Alzheimer's Disease.Curr Issues Mol Biol. 2022 Aug 9;44(8):3533-3551. doi: 10.3390/cimb44080243. Curr Issues Mol Biol. 2022. PMID: 36005139 Free PMC article.
References
-
- Aoki K., Nakamura T., Fujikawa K., Matsuda M. (2005). Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Mol. Biol. Cell 16 2207–2217. 10.1091/mbc.e04-10-0904 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

