Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes From Ocimum sanctum Linn. Against Root Rot Pathogen Fusarium oxysporum in Pisum sativum
- PMID: 35237287
- PMCID: PMC8884270
- DOI: 10.3389/fpls.2022.813686
Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes From Ocimum sanctum Linn. Against Root Rot Pathogen Fusarium oxysporum in Pisum sativum
Abstract
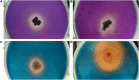
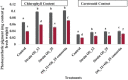
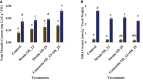
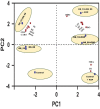
The present study demonstrates plant growth promotion and induction of systemic resistance in pea (Pisum sativum) plant against Fusarium oxysporum f.sp. pisi by two bacterial endophytes, Pseudomonas aeruginosa OS_12 and Aneurinibacillus aneurinilyticus OS_25 isolated from leaves of Ocimum sanctum Linn. The endophytes were evaluated for their antagonistic potential against three phytopathogens Rhizoctonia solani, F. oxysporum f. sp. pisi, and Pythium aphanidermatum by dual culture assay. Maximum inhibition of F. oxysporum f. sp. pisi was observed by strains OS_12 and OS_25 among all root rot pathogens. Scanning electron microscopy of dual culture indicated hyphal distortion and destruction in the case of F. oxysporum f. sp. pisi. Further, volatile organic compounds (VOCs) were identified by gas chromatography-mass spectrometry (GC-MS). The GC-MS detected eight bioactive compounds from hexane extracts for instance, Dodecanoic acid, Tetra decanoic acid, L-ascorbic acid, Trans-13-Octadecanoic acid, Octadecanoic acid. Both the endophytes exhibited multifarious plant growth promoting traits such as indole acetic production (30-33 μg IAA ml-1), phosphate solubilization, and siderophore and ammonia production. Pot trials were conducted to assess the efficacy of endophytes in field conditions. A significant reduction in disease mortality rate and enhancement of growth parameters was observed in pea plants treated with consortium of endophytes OS_12 and OS_25 challenged with F. oxysporum f.sp. pisi infection. The endophytic strains elicited induced systemic resistance (ISR) in pathogen challenged pea plants by enhancing activities of Phenylalanine ammonia lyase (PAL), peroxidase (PO), polyphenol oxidase (PPO), ascorbate oxidase (AO), catalase (CAT) and total phenolic content. The endophytes reduced the oxidative stress as revealed by decrease in malondialdehyde (MDA) content and subsequently, lipid peroxidation in host plant leaves. Robust root colonization of pea seedlings by endophytes was observed by scanning electron microscopy (SEM) and fluorescence microscopy. Thus, plant growth promoting endophytic P. aeruginosa and A. aneurinilyticus can be further exploited through bio-formulations for sustainable protection of crops against root rot diseases as bio-control agents.
Keywords: Fusarium root rot; biological control; endophytes; induced systemic resistance; plant growth promotion; volatile compounds.
Copyright © 2022 Gupta, Pandey and Sharma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Agarwal H., Dowarah B., Baruah P. M., Bordoloi K. S., Krishnatreya D. B., Agarwala N. (2020). Endophytes from Gnetum gnemon L. can protect seedlings against the infection of phytopathogenic bacterium Ralstonia solanacearum as well as promote plant growth in tomato. Microbiol. Res. 238:126503. 10.1016/j.micres.2020.126503 - DOI - PubMed
-
- Agarwal M., Dheeman S., Dubey R. C., Kumar P., Maheshwari D. K., Bajpai V. K. (2017). Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 205 40–47. 10.1016/j.micres.2017.08.012 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

