miR-672-3p Promotes Functional Recovery in Rats with Contusive Spinal Cord Injury by Inhibiting Ferroptosis Suppressor Protein 1
- PMID: 35237382
- PMCID: PMC8885177
- DOI: 10.1155/2022/6041612
miR-672-3p Promotes Functional Recovery in Rats with Contusive Spinal Cord Injury by Inhibiting Ferroptosis Suppressor Protein 1
Abstract
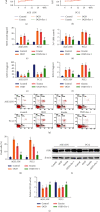
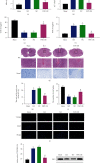
Aberrantly expressed microRNAs (miRNAs) after spinal cord injury (SCI) participate in diverse biological pathways and processes, including apoptosis, inflammation, oxidative stress responses, peroxidation, and ferroptosis. This study was aimed at exploring the mechanisms underlying miRNA-mediated ferroptosis in an SCI rat model. In the present study, a T10 weight-dropping SCI model was established and miRNA profiling was used to detect miRNA expression profiles post-SCI. Basso-Beattie-Bresnahan scores and inclined plane test, hematoxylin and eosin (HE) and Nissl staining, immunohistochemistry and immunofluorescence, western blotting, cell viability, and Annexin V/7-aminoactinomycin D (7-AAD) assays were used to evaluate locomotor activity, histological changes in the injured spinal cords, neuronal ferroptosis, ferroptosis suppressor protein 1 (FSP1) expression, and cell death, respectively. It was observed that many miRNAs were differentially expressed after SCI, and miR-672-3p, which increased significantly, was selected after cross-referencing with predicted target miRNAs. The luciferase reporter assay demonstrated that miR-672-3p downregulated FSP1, a glutathione-independent ferroptosis suppressor, by binding to its 3' untranslated region. Oxygen and glucose deprivation- (OGD-) treated PC12 and AGE1.HN cells were treated with miR-672-3p mimics or inhibitors in vitro. The effect of miR-672-3p mimics or inhibitor on OGD-PC12/AGE1.HN ferroptosis was evaluated by flow cytometry, immunohistochemistry, immunofluorescence, and western blotting. The miR-672-3p mimics promoted ferroptosis after SCI, whereas the miR-672-3p inhibitor inhibited this process. Rats with SCI treated with miR-672-3p mimics or inhibitor showed similar results in vivo. Furthermore, the ferroptosis-related changes caused by SCI or miR-672-3p were reversed by overexpression of FSP1 lentivirus in vivo and in vitro. These results indicated that sh-miR-672-3p exerted a neural restoration effect in vivo and in vitro by inhibiting ferroptosis via the FSP1 pathway.
Copyright © 2022 Fang Wang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

