Trace Level Quantification of 4-Methyl-1-nitrosopiperazin in Rifampicin Capsules by LC-MS/MS
- PMID: 35237562
- PMCID: PMC8883033
- DOI: 10.3389/fchem.2022.834124
Trace Level Quantification of 4-Methyl-1-nitrosopiperazin in Rifampicin Capsules by LC-MS/MS
Abstract
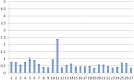
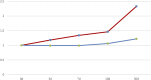
Rifampicin is a first-line anti-tuberculosis drug. However, in August 2020, the presence of 1-methyl-4-nitrosopiperazine (MNP), a nitrosamine impurity, was detected by the United Stated Food and Drug Administration (US FDA) in rifampicin capsules. Consequently, the development of efficient methods for the detection of MNP is an important objective. In this study, the MNP present in rifampicin capsules was detected using LC-MS/MS. A total of 27 batches from nine manufacturers in the Chinese market were tested, with MNP (0.33-2.36 ppm) being detected in all samples at levels exceeding the maximum acceptable intake limit of 0.16 ppm initially set by the FDA. However, after considering the associated benefits and risks, the FDA-approved limit was revised to 5 ppm; hence, all the samples examined herein exhibited MNP levels well below the required limit. Furthermore, the results of forced degradation experiments suggest that MNP is formed by the thermal degradation of rifampicin.
Keywords: LC-MS/MS; anti-tuberculosis; genotoxic impurity; nitrosamine impurity; rifampicin.
Copyright © 2022 Tao, Tian, Liu, Yao and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Committee for Medicinal Products for Human Use, (CHMP) (2006). (CPMP/SWP/5199/02. EMEA/CHMP/QWP/251344/2006): Guideline on the Limits of Genotoxic Impurities. London: European Medicines Agency EMEA.
-
- ICH (2020). International Conference on Harmonization (ICH) Guidelines, M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Available at: https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf
-
- Kecili R., Billing J., Leeman M., Nivhede D., Sellergren B., Rees A., et al. (2013). Selective Scavenging of the Genotoxic Impurity Methyl P-Toluenesulfonate from Pharmaceutical Formulations. Separat. Purif. Technol. 103, 173–179. 10.1016/j.seppur.2012.09.028 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

