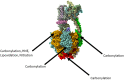Mitochondrial ATP Synthase is a Target of Oxidative Stress in Neurodegenerative Diseases
- PMID: 35237666
- PMCID: PMC8882969
- DOI: 10.3389/fmolb.2022.854321
Mitochondrial ATP Synthase is a Target of Oxidative Stress in Neurodegenerative Diseases
Abstract
The mitochondrial ATP synthase is responsible for the production of cellular ATP, and it does so by harnessing the membrane potential of the mitochondria that is produced by the sequential oxidation of select cellular metabolites. Since the structural features of ATP synthase were first resolved nearly three decades ago, significant progress has been made in understanding its role in health and disease. Mitochondrial dysfunction is common to neurodegeneration, with elevated oxidative stress a hallmark of this dysfunction. The patterns of this oxidative stress, including molecular targets and the form of oxidative modification, can vary widely. In this mini review we discuss the oxidative modifications of ATP synthase that have been observed in Alzheimer's disease, Parkinson's disease, and Huntington's disease. Oxidative modifications of ATP synthase in Alzheimer's disease are well-documented, and there is a growing body of knowledge on the subject in Parkinson's disease. The consideration of ATP synthase as a pharmacological target in a variety of diseases underlines the importance of understanding these modifications, both as a potential target, and also as inhibitors of any pharmacological intervention.
Keywords: ATP synthase; mitochondria; neurodegenarative disease; oxidative phoshorylation; oxidative stress.
Copyright © 2022 Ebanks and Chakrabarti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources