Evidence for H-bonding interactions to the μ-η2:η2-peroxide of oxy-tyrosinase that activate its coupled binuclear copper site
- PMID: 35237779
- PMCID: PMC8966618
- DOI: 10.1039/d2cc00750a
Evidence for H-bonding interactions to the μ-η2:η2-peroxide of oxy-tyrosinase that activate its coupled binuclear copper site
Abstract
The factors that control the diverse reactivity of the μ-η2:η2-peroxo dicopper(II) oxy-intermediates in the coupled binuclear copper proteins remain elusive. Here, spectroscopic and computational methods reveal H-bonding interactions between active-site waters and the μ-η2:η2-peroxide of oxy-tyrosinase, and define their effects on the Cu(II)2O2 electronic structure and O2 activation.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

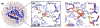
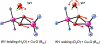
References
-
- Hirota S, Kawahara T, Lonardi E, de Waal E, Funasaki N and Canters GW, J. Am. Chem. Soc, 2005, 127, 17966–17967. - PubMed
-
- Magnus KA, Hazes B, Ton-That H, Bonaventura C, Bonaventura J and Hol WGJ, Proteins, 1994, 19, 302–309. - PubMed
-
- Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H and Sugiyama M, Journal of Biological Chemistry, 2006, 281, 8981–8990. - PubMed
-
- Brown JM, Powers L, Kincaid B, Larrabee JA and Spiro TG, J. Am. Chem. Soc, 1980, 102, 4210–4216.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

